
Reversibility of chemical reactions. Chemical equilibrium. Conditions for shifting the chemical equilibrium according to the principle of Le Chatelier - CHEMICAL REACTIONS - LESSON PLANS for CHEMISTRY 11 class - lesson plans - lesson plans-author's lessons-plan-lesson summary - chemistry
Lesson objectives: to consolidate the concepts of "reversibility" and "irreversibility" of chemical reactions; to generalize and to deepen students ' knowledge about chemical equilibrium, the equilibrium constant, the principle of Le Chatelier and be able to apply it to the displacement of chemical equilibrium; to give an idea of the importance of knowledge about chemical equilibrium in the production and in the nature and development of skills in solving computational problems with the use of the term "equilibrium constant".
Basic concepts: reversible and irreversible chemical reactions, chemical equilibrium, equilibrium concentrations, equilibrium constant, reaction rate, Le Chatelier principle.
Equipment: solutions ofFeCl 3; KNCS-potassium cyanide; KCl-crystal; starch paste; test tubes, water, alcohol lamp, matches, holder.
Lesson progress
I. Front-end survey
1. Determination of the rate of chemical reaction.
2. Formula expressions of speed and units of speed:
a) homogeneous reaction;
b) heterogeneous reaction.
3. List the factors that affect the rate of chemical reaction.
4. For example, the reaction of zinc (g) with hydrochloric and acetic acids to explain the effect
a) the nature of the reacting substances on the reaction rate;
b) temperature on the rate of chemical reactions: formulae of van't Hoff;
C) the contact surfaces of reacting substances for the rate of chemical reaction; compare the rate of reaction of iron in the form of powder and iron in the form of chips with hydrochloric acid.
5. How does the rate of chemical reaction depend on the concentration? The law of active masses of the reacting substances. Make a kinetic equation of a chemical reaction.
Example.
![]()
6. What substances are called catalysts? Inhibitors? What is the difference between their effect on the speed of a chemical reaction? The importance of catalysts and inhibitors in production, in the life of living organisms.
7. What do you need to know about a chemical reaction to determine its rate? Select the correct answers.
a) type of reaction;
b) composition and aggregate state of the initial products and final products of the reaction;
C) the concentration of all initial products of the reaction;
d) the concentration of one reaction product after a certain period of time and the value of the time interval;
e) temperature coefficient, temperature change over a certain period of time;
e) contact surface area; presence of a catalyst.
The answers are pre-recorded on the back of the Board.
Answers to questions in the front-end survey
b) the composition and aggregate state of the initial and final reaction products.
Find
out if the reaction is heterogeneous or homogeneous, because the formulas for
calculating the reaction rate are different for them, for a homogeneous
reaction —![]() for a
reaction in General form AA + BB = C.
for a
reaction in General form AA + BB = C.
When calculating the velocity according to the law of acting masses, do not take into account substances in the solid state, as well as immiscible liquids.
![]()
C) the concentration of all initial reaction products, since the reaction rate is directly proportional to the concentration of reacting substances (for homogeneous reactions).
d)
the speed is determined by the formula ![]() ΔC
is determined by the difference in the initial and final concentration of one
of the reactants. Δt — the time period for changing the concentration.
ΔC
is determined by the difference in the initial and final concentration of one
of the reactants. Δt — the time period for changing the concentration.
e)
the temperature coefficient y for each reaction is known, it is necessary for
calculating the change in the reaction rate as a result of an increase or
decrease in temperature according to the Vanth-Hoff law ![]()
f)
the contact surface of reactants is taken into account in heterogeneous
reactions according to the formula ![]()
The more fragmented a substance is, the smaller the unit area, and the faster the reaction rate. Catalyst-accelerator. A substance that ultimately increases the reaction rate, but is not consumed by itself. The action of an inhibitor is the opposite of that of a catalyst.
Catalysts are substances that change the reaction mechanism and direct it in a more favorable direction of energy-lessEa. They do not change qualitatively or quantitatively.
II. Independent work
|
Option I |
Option II |
|
1. Make an expression of the speed of a chemical reaction according to the law of acting masses. |
|
|
|
|
|
2. How will the response rate change based on the task condition, decrease or increase? |
|
|
|
|
|
3. Calculate the speed |
|
|
of a homogeneous reaction |
a heterogeneous reaction |
|
by the condition: |
|
|
|
|
|
Perform a self-check. |
|
Answers to self-study questions
Option I
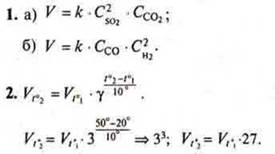
Answer: the reaction speed will increase by 27 times.
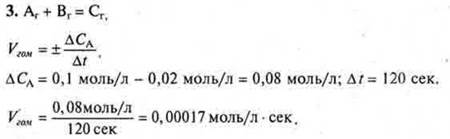
Answer: VGOM.= 0.00017 mol/l · sec.
Option II
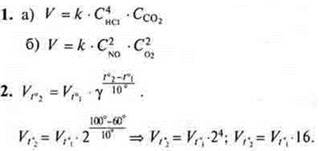
Answer: the reaction speed will increase by 16 times.
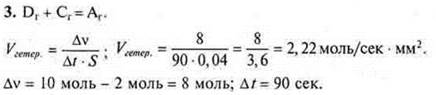
Answer: Vhetero.= 2.22 mol/sec · mm2.
II. Learning new material
Plan of presentation:
1. Reactions are irreversible and reversible. Signs of irreversibility.
2. Chemical equilibrium. The constant of chemical equilibrium.
3. Factors that cause chemical equilibrium mixing. The Le Chatelier Principle. Experiment.
4. Solving computational problems of the topic.
Task: from the proposed equations of chemical reactions to choose the reactions:
a) irreversible;
b) reversible.
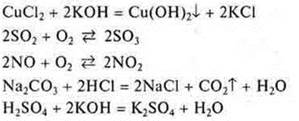
Irreversible reactions have signs of irreversibility:
![]() —
there's been a fallout.
—
there's been a fallout.
![]() — a
weak electrolyte was formed, which decomposes into water and carbon dioxide.
— a
weak electrolyte was formed, which decomposes into water and carbon dioxide.
![]() —
water was formed — a very weak electrolyte.
—
water was formed — a very weak electrolyte.
The rest of the reactions under the same conditions can occur both in the forward and in the reverse direction. They are reversible.
![]()
It is known that most reactions are reversible. If we are talking about a closed system, then under certain conditions, the irreversible reaction becomes reversible.
Example:
a)
in an open system, the reaction![]() is
irreversible;
is
irreversible;
b) in a closed system — it becomes reversible:
![]()
Let's look in more detail at the processes occurring in reversible reactions for a conditional reaction.
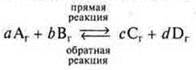
According to the law of acting masses, we will determine the rates of forward and reverse reactions.
![]() because
over time WithA and CBdecreases, then the rate of direct
reaction decreases. Since the direct reaction results in the formation of
products C and D, and the reaction is reversible, the speed of the reverse
reaction begins to increase.
because
over time WithA and CBdecreases, then the rate of direct
reaction decreases. Since the direct reaction results in the formation of
products C and D, and the reaction is reversible, the speed of the reverse
reaction begins to increase.
![]()
There comes a point when the rates of forward and reverse reaction become equal: VPR. = VOBR.and the concentrations of reactants and end products of the reaction remain unchanged. They are called equilibrium concentrations. This equilibrium in the system is called a moving or dynamic one. We should consider Fig. 34 p. 142 of the textbook, which shows graphs of changes in the concentration and rates of reactions of irreversible and reversible.
Reactions are irreversible — with increasing time, the concentrations of the initial substances decrease to 0, and the concentration of the final products increases. The reaction rate decreases to 0. the Reactions are reversible — the concentrations of the initial substances decrease, while the concentrations of the final products of the reaction increase in a certain period of time. The speed of the direct reaction decreases, and the speed of the reverse reaction increases over the same period of time. There is a moment of equalization of the rates of forward and reverse reactions, and the concentrations of the initial products and final products of the reaction remain unchanged.
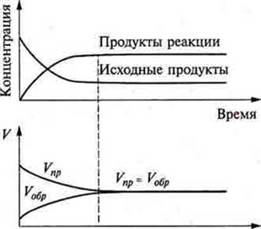
The state of a system at which the rate of direct reaction is equal to the rate of reverse reaction is called chemical equilibrium.
The state of chemical equilibrium can be given a quantitative characteristic by applying the law of acting masses, through the chemical equilibrium constant tothe equation of equalmass .— constant value for a given reversible reaction. It shows the ratio between the concentrations of the reaction products (numerator) and the initial substances (denominator), which is established at equilibrium.
In the case of a chemical reaction in General form
![]() the
equilibrium concentrations of substances are [A]; [B]; [C]; [D].
the
equilibrium concentrations of substances are [A]; [B]; [C]; [D].
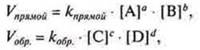
because at the moment of chemical equilibrium
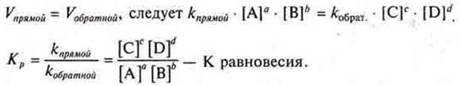
If Kis equal. > 1-concentrations of reaction products predominate over the concentration of starting products in a direct reaction, the system is directed towards →
If Kis equal. < 1, the concentration of the initial reaction products dominated by the concentration of end products of the reaction, the system is directed toward the reverse reaction ←
If
Kis equal. = 1, the concentration of the initial reaction
products and the final ones remain unchanged, since the rates of forward and
reverse reactions are equal, and chemical equilibrium has arrived ![]()
In the case of heterogeneous reactions, the expression of the equilibrium constant includes concentrations of substances that are in the gas and liquid phase.
Example: ![]()
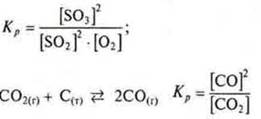
The value of the equilibrium constant depends on the nature of the reacting substances and temperature. The catalyst lowersEactivatesboth the forward and reverse reaction by the same amount and only accelerates the onset of equilibrium.
The state of chemical equilibrium can be maintained for a long time under unchanged external conditions: temperature, concentration of initial substances or final pressure products (if gases are involved in the reaction).
If you change these conditions, you can move the system from one equilibrium state to another that meets the new conditions.
Such a shift is called a displacement or shift of balance. Displacement control can be predicted using Le Chatelier's principle, 1884.
When the equilibrium system is influenced from the outside (to change the concentration, pressure, temperature), the equilibrium shifts in the direction of the reaction (direct or reverse) that weakens this effect.
1) Change in concentration:
a) if we increase from the final products, the balance shifts towards the formation of initial products, i.e. the reverse reaction prevails:
![]()
b) we increase the concentration of initial products, the balance shifts towards the formation of final products, a direct reaction prevails:
![]()
C) when the concentration of end products decreases, the equilibrium reaction shifts towards their formation, and the direct reaction prevails:
![]()
d) when the concentration of initial reaction products decreases, the equilibrium shifts towards the formation of initial reaction products, the reverse reaction prevails:
![]()
2) Influence of pressure change:
a) when the pressure increases, the equilibrium shifts in the direction of the reaction, in which the volume of formed gaseous products decreases:
R^ a, in the direction of the least amount of;
b) when the pressure decreases, the equilibrium shifts in the direction of the reaction, in which the volume of formed gaseous products increases:
P↓ — in the direction of the maximum volume.
Example. 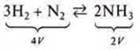
a)
P↑ — direct reaction![]() (direct
P↑);
(direct
P↑);
b)
P↓ — reverse reaction![]() (reverse
P↓);
(reverse
P↓);
C) if the volumes of gaseous products are the same in both the forward and reverse reaction — the change in pressure does not cause a shift in the equilibrium.
Example: 
3) Influence of temperature change:
a) when the temperature rises, the chemical equilibrium shifts towards the endothermic reaction.
t ° ↑ - to the side - Q;
b) when the temperature drops, the chemical equilibrium shifts towards the endothermic reaction.
t ° ↓ - to the side + Q;
Example. ![]()

The principle of Le Chatelier is also applicable to the processes of evaporation, condensation, melting, and crystallization. When producing the most important chemical products, they take into account the peculiarities of chemical reactions, especially reversible ones, and find optimal conditions that ensure maximum product yield.
Further, students are shown experiments proving the possibility of shifting the chemical equilibrium by applying factors:
a) temperature change.
Add solution I2 to the starch paste. There is a blueness, because the starch macromolecule-amylose-reacts with I2, forms a colored complex — an amylose molecule in the form of a spiral, surrounded by iodine molecules. 2There are six glucose bases around each I2 molecule. When heated, this complex is destroyed and the color disappears when cooled — the complex is formed again and the color is restored.
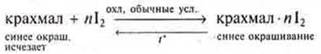
b) changes in the concentration of initial and final products of the reaction.
In four test tubes add 2 ml of water and 1-2 drops of KNCS
(rhodanide)
of potassium; and 1-2 drops![]() of the
solution became light red in all test tubes.
of the
solution became light red in all test tubes.
![]()
Add 2-3 drops of FeCl 3 to test tube # 13 — the color increases, there is a direct reaction → Vdirect > > VOBR.
Add 2-3 drops of KNCS to test tube # 2 — the color increases, there is a direct reaction → Vdirect > > VOBR.
In test tube # 3 add CSl-color disappears, there is a reverse reaction ← Vdirect < VOBR.
Test tube # 4 - for comparison.
III. Generalizations and conclusions
Thus, in this lesson, we have studied in more depth the chemical equilibrium that can occur in reversible chemical reactions, and also got an idea of the factors that cause the shift of chemical equilibrium in the direction of a direct or reverse reaction, experimentally confirmed this.
IV. Homework assignment
§ 14, # 1-4, problems # 7, 8.
V. Fixing and solving calculation problems
Task. № 1(1) § 14.
![]()
![]()
![]() towards
a lower volume.
towards
a lower volume.
Task. № 2 (1) § 14.
![]()
![]() towards
a lower volume.
towards
a lower volume.
Task. 5 (1) § 14.
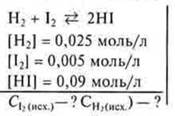
![]()
1) γH2 — spent to obtain 0.09 HI
![]()
![]()
2) γI2 — spent to obtain 0.09 NI
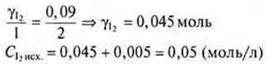
Answer: WithI2 ex. = 0.05 (mol/l); WithH2 (ex.)= 0.07 (mol/l).
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.