
Theory of electrolytic dissociation. Properties of electrolyte solutions-DISPERSED SYSTEMS. SOLUTIONS. PROCESSES OCCURRING in SOLUTIONS - LESSON DEVELOPMENTS in CHEMISTRY grade 11 - lesson developments-lesson developments - author's lessons - lesson plan-lesson summary - chemistry
The purpose of the lesson: to deepen and generalize knowledge, the basic concepts of electrolytic dissociation; to teach them to apply them in the formulation of dissociation equations, ion exchange reactions; to give an idea of the universality of the theory of electrolytic dissociation, its application to inorganic and organic chemistry.
Basic concepts: electrolytes, non-electrolytes, dissociation, Association, hydrated ions, cations, anions, strong, weak electrolytes, degree of electrolytic dissociation, dissociation constant.
Equipment: totransport printed out the test questions on the table, reference the abstract of the lecture.
Lesson progress
I. Organizational moment
The lesson combined. The lesson provides independent work at the time of learning a new material — a test task, during the discussion of which information on the theory of electrolytic dissociation is summarized.
Solution of computational problems for quantitative characterization of solutions.
II. Independent work
|
Option I |
Option II |
|
|
|
Problem solving options
Option I
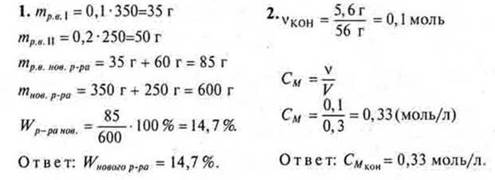
Option II
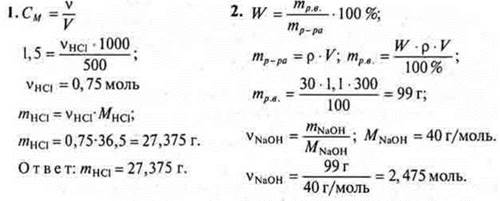
II. Learning new material
Plan of presentation
1. students ' Work with the tasks of the kodotransporant test on кодотранспорантеthe topic "Electrolytic dissociation" in order to reproduce students ' knowledge.
2. Basic concepts of the theory of electrolytic dissociation:
a) electrolyte and non-electrolyte;
b) strong and weak electrolytes;
C) degree of dissociation, dissociation constant.
3. Dissociation of acids, salts, bases.
4. Reaction.AI IOx exchange.
In the 9th grade, we studied the topic of TED. In this lesson, we will remember the key theoretical issues of this topic, " strengthen the basic concepts of TED, as well as the ability to create equations for the dissociation of electrolytes andreactions of ion exchange.
First, we will work with the test on the codotransport (or: the test questions are printed in the calculation of one copy per Desk).
1. What substances are called electrolytes, and a nonelectrolytes?
a) choose electrolytes: nitrogen, potassium chloride (p-p), nitric acid (P↑), carbon dioxide, sucrose;
b) choose non-electrolytes: glucose, zinc chloride, alcohol.
2. Define dissociation. Is this the reverse process?
3. Correlate the dissociation patterns of a substance:
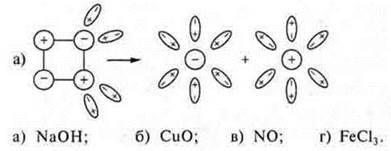
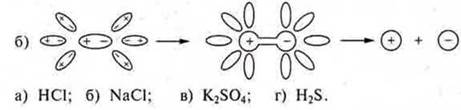
What shows the degree of electrolytic dissociation?
Choose the formula of a) strong electrolytes, b) weak electrolytes:
Fe(OH)3; HNO3; VASO3; CA3(RO4)2; Na2S; CON; N3RO4; NH4OH.
4. Choose and name a) cations and b) anions:
![]()
5. What substances in the dissociation to form silicate-ions?
a) CaSiO3; b) Na2SiO3; C) H2SiO3; d) SiO2.
6. What substances form during the dissociation of the cations of magnesium?
a) MgCl2b) Mg3(PO4)2; C) MgO; d) Mg(NO3)2.
7. in the solutions of which substances is there an excess:
a) the cation of hydrogen;
b) hydroxide anion of the substance: salts? acids? alkalis?
8. define acids, salts, and alkalis from the point of view of the theory of electrolytic dissociation.
9. From the suggested formulas of the substances Fe2(SO4)2, Ba(HE)2, Na2SiO3, H2SO4 select the substances that dissociate according to the schemes:
![]() —
metal cation;
—
metal cation;
![]() —
acid residue;
—
acid residue;
![]()
10. That shows the degree of electrolytic dissociation?
11. The electrolyte solutions:
a) NaOH and H2SO4;
b) Ba(NO3)2 and NSl;
C) AgNO3 and NaBr;
d) Na2CO3 and HCl.
Are there possibleionicexchange reactions between them?
12. Correlate the reduced ionic equations of the reactions:
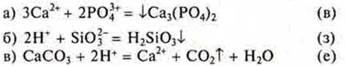
![]()
with the left side of the molecular equations:
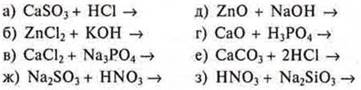
During the discussion of answers to questions, the teacher introduces additions to some questions.
1. Electrolytes are substances whose solutions and melts conduct electric current. As a rule, these are compounds with an ionic bond and with a covalent polar bond:
KCl — ion bond; HNO3-- between H+and NO3-ion bond.
Non-electrolytes are substances whose solutions and melts do not conduct electric current (organic compounds, gases):
C6H12O6-glucose, C2H5IT is ethanol, alcohol.
2. Dissociation — the breakdown of an electrolyte into ions when dissolved or melted. This is a reversible process.
The process that is the reverse of dissociation is called Association.
3. The mechanism of dissociation:
a) substances with an ionic bond:
![]()
b) substances with a polar covalent bond:
"water
dipole» ![]() polarizes
the bond, it becomes ionic:
polarizes
the bond, it becomes ionic:
![]()
4. Cations: Na+- sodium cation; CA2+- calcium cation; NH4+— ammonium cation.
Anions: MPO4-- permanganate-anion; PO43 -- phosphate-anion; NO2-- nitrite-anion; HCO3-- bicarbonate-anion.
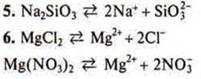
7. a) in acid solutions:
![]()
b) in solutions of alkalis:
![]()
8. Acids — electrolytes, in solutions of which only hydrogen cations are represented as cations:
![]()
Alkalis are electrolytes that are represented as anions in solutions. Only hydroxide anions:
![]()
Salt — electrolytes in solutions which are formed during the dissociation of the cations of the metal (or ammonium ion) and anions of acidic residues.
9.![]() - base
- base
![]() —
acid
—
acid
![]() —
medium salt
—
medium salt
![]() —
medium salt
—
medium salt
10. the Degree of electrolytic dissociation a shows the percentage of molecules dissociated to the total number of electrolyte molecules:
![]()
a) strong electrolytes, α → 1. HNO3; Na2S; CON. In their solutions the equilibrium is completely shifted towards a direct reaction:
![]()
b) weak electrolytes, α → 0. VASO3; Fe(OH)3; CA3(RO4)2; N3RO4; NH4OH; N2CO3.
Many dissociate in steps.
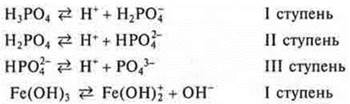
In solutions of weak electrolytes, the equilibrium shifts towards the formation of molecules, i.e. towards the reverse reaction.
For the characteristics of weak electrolytes are used, the dissociation constant for each stage

The dissociation constant is the ratio of product of equilibrium concentrations of cations and anions, elevated to the degree of the coefficients of the equilibrium concentration to concentration netradicionnyh molecules, Kd depends on the nature of electrolyte, nature of solvent and temperature, but is independent of concentration. Sometimes Kdis calculated using the concentration and degree of dissociation:
![]()
Example:
at t° = 25° C

So, themoreKD , the easier it is to break up the electrolyte into ions, the more ions in its solution, the stronger the electrolyte.
11.ion exchangeReactions are irreversible if a precipitate, gas, water, or weak electrolyte is formed:
![]() - the
molecular equation of the form
- the
molecular equation of the form
![]() -
General ionic appearance
-
General ionic appearance
![]() -
weak water electrolyte; equation of the short ionic form
-
weak water electrolyte; equation of the short ionic form
![]() — the
ion exchange reaction doesn't make sense
— the
ion exchange reaction doesn't make sense
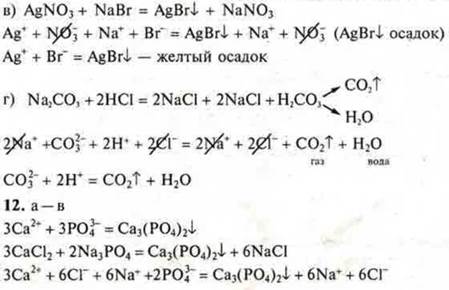
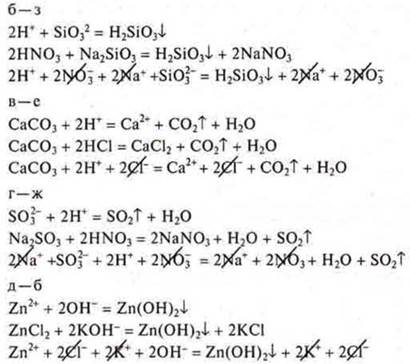
Ions can enter into redox reactionswith atoms and molecules.
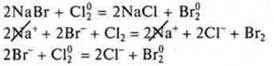
Generalizations and conclusions from the lesson.
IV. Homework assignment
§ 15, pp. 148-151, 154-155.
N° 1-2 (y). perform the Following tasks on a separate sheet of options for evaluation.
|
Option I # 3 # 8 (a, b, C, g) # 9 (a, b, d) # 10 (a, b, b, h) |
Option II # 4 # 8 (d, d, e, h) # 9 (b, e, b) # 10 (d, d, e, W) |
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.