
The state of electrons in an atom
Lesson objectives: to consolidate knowledge of the concepts of "electron cloud", "orbital", "energy level", "sublevel energy"; to form a view on the quantum numbers characterizing the energy of the electron in the atom, shapes of orbitals, their number and arrangement in space around the nucleus; to teach how to describe the state of an electron in an atom using quantum numbers.
Basic concepts: electron cloud, orbital, energy level, energy sublevel quantum number — main, orbital (side), magnetic, spinovoe (with 2 hours of chemistry a week only to familiarize students with the main quantum number and learn to use it to characterize the state of an electron in an atom; however, if you have the opportunity to acquaint students with all quantum numbers to use it, it will help to more correctly and much clearer in the future to explain to the students the electronic configuration of atoms).
Equipment: pshe D. I. Mendeleev.
Lesson progress
I. Organizational moment
The teacher together with the students checks the correctness of homework.
The rest of the students are given a front-line survey on the following questions:
1. List all the physics discoveries of the end of the XIX—beginning of the XX century that confirm the complexity of the structure of the atom.
2. What models of the structure of the atom do you know? What is their failure?
3. What are the postulates proposed N. Bor? Why is his theory considered the most important stage in the development of the idea of the structure of the atom?
4. Explain the dual nature of micro-world particles.
5. what is the essence of the proton-neutron theory of the nucleus of an atom?
6. Specify the elementary particles of an atom, their mass and charge.
7. How to determine the composition of an atom and the charge of its nucleus based on the position of an element in the Mendeleev PSE?
These questions can be written in totransparency or in printed form to give to students. It all depends on the availability of TCO in the school.
Next, we check the homework completed by students on the gloss.
II. Learning new material
Plan of presentation
1. The motion of an electron in the atom. Electron cloud. The electron orbital s -, p-, d -, f -.
2. The energy of the electron. Quantum numbers: main, orbital (secondary), magnetic, and spin. Characterization of the electron state in an atom by quantum numbers.
3. Energy level, energy sublevel. The physical meaning of the period number.
4. Characteristics of the state of electrons in the atom of element No. 8.
We repeat what we already know:
the theory dealing with the study of the movement of particles is called quantum mechanics;
— an electron exhibits both particle and wave properties;
— according to quantum mechanics, the movement of an electron around the nucleus of an atom cannot be considered simply as a mechanical movement.
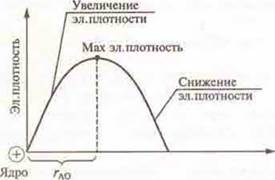
According to this theory, an electron can be located at any point near the nucleus of an atom, but the probability of its staying at different points varies. Thus, if we could observe an electron in an atom, we would see that it is more frequent in some places and less frequent in others. Therefore, an electron moving in an atom forms a so-called electron cloud. An electron cloud is a volume of space relative to the nucleus in which all the mass and charge of an electron are concentrated. The electronic density of the electronic cloud is distributed unevenly. In the core, it is equal to zero. As you move away from the core, it increases, and then decreases. The volume of space relative to the nucleus, in which about 90% of the electron density is concentrated, is called the atomic orbital (AO). The distance from the nucleus to the maximum electron density is called the atomic radius. The energy of an atomic orbital depends on its radius. The larger the radius of the atomic orbital, the greater the energy. Atomic orbitalsthat have the same energy reserve and the same radius form the energy level in the atom. The period number of a chemical element in D. I. Mendeleev's PSE corresponds to the number of energy levels in an atom.
Example:
![]()
Example: period VI Elements in an atom have six energy levels. However, electrons of the same energy level may differ from each other in their binding energy to the nucleus of an atom. At the energy level, sub-levels arise. The number of sublevels at the energy level corresponds to the number of the energy level.
Example: Number of energy level III, therefore, it is possible to open three sublevels.
An important corollary from quantum mechanics is that: because theentire set of complex movements of an electron in an atom is characterized by energy numbers, which are called quantum numbers.
n is the main quantum number, determines the total energy of an electron of a given energy level, and takes the values of integers of a natural series— 1, 2, 3, ..., ∞.
Further, it is possible to explain the state of an electron in an atom by taking into account only the main quantum number. You need to work with the table in textbook # 1. This table allows you to determine the number of energy levels and sublevels in an atom of a chemical element, taking into account only the main quantum number, calculate the number of orbitals in the level and sublevel, and calculate the maximum number of electrons at the energy level and sublevel.
Example: n = 3; in the third energy level — sublevels — 3: 3s, 3p, 3d; number of orbitals in level: n2— 9: sublevels: 3s — 1, 2P — 3, 3d — 5; maximum number of electrons in the level 2n2— 18; on the sublevel: the s — 2, R — 6, d — 10.
Taking into account the fact that in the further study of the topic, especially the question related to the electronic configurations of atoms of chemical elements, it is impossible to do only the main quantum number, it is necessary to familiarize students with other quantum numbers in this lesson. Moreover, many questions of the use tests require an answer using the knowledge of quantum numbers.
So, the state of an electron in an atom is characterized by quantum numbers:
n is the main quantum number (its characteristic is already known to us);
l is a side (orbital) quantum number. Sublevels of the energy level are characterized by a side quantum number. It depends on the main quantum number and takes values from 0 to n - I.
The secondary quantum number characterizes the shape of the atomic orbital and specifies its energy by the formula E = n + l.
1. At l = 0, a sublevel s opens with an s-orbitalwhose shape is spherical.
2. At l = 1, the sublevel p opens with p-orbitals, the shape of which resembles a three-dimensional eight.
3. When l = 2 opens sublevel d with d-orbitals, the shape of which resembles a volume petal and a more complex volume eight.
4. At l = 3, a sublevel f opens with f-orbitals, having a more complex shape.
The energy level number corresponds to the number of sublevels. For n = 3, there are three sublevels; for n = 2, there are two sublevels.
The number of orbitals at the sublevel is determined by the m-magnetic quantum number. The magnetic quantum number determines the distribution of orbitals in the magnetic field of the nucleus. it depends on the orbital quantum number and takes values from 0 to l-1; m = 2l + 1.
Example: for l = 0, ml = 0, орбитальthere is one orbital; for l = 1, m = -1, 0, +1, there are three orbitals.
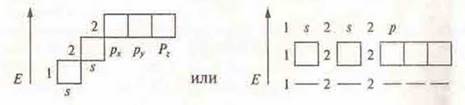
It should be noted that all orbitals are located symmetrically in space:
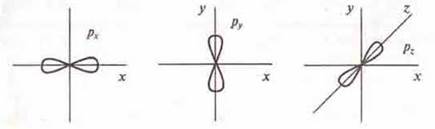
Atomic orbitals can be represented as cells or dotted lines:
Using
a cell![]() or
dotted line
or
dotted line ![]()
s-sublevel:![]() or
or ![]()
p-sublevel:![]() or
or ![]()
d-sublevel: or
or ![]()
f-sublevel: or
or 
The spin quantum number s is independent. This number is a quantum property of an electron that has no classical analogues. Spin is the electron's own moment of momentum, which is not related to motion in space. For all electrons, the absolute spin value is 1/2. The projection of spin on the axis (magnetic spin number mscan have only two values: +1/2 or -1 / 2, because the electron spin is a constant value.)
Conclusion: The state of an electron in an atom is characterized by quantum numbers: n — the main quantum number, l — the side quantum number, ml — the magnetic orbital quantum number,ms-the magnetic spin quantum number.
Knowing the quantum numbers of an electron, you can describe the energy, number of orbitals, their shape and location in space.
III. Consolidation of the studied material
Task: Describe the state of electrons in the atom of chemical element # 8, using all quantum numbers.
Answer: Element # 8 is oxygen, the charge of the nucleus of an atom is +8, and there are 8 electrons in an atom. Oxygen is located in the II period, n = 2. therefore, in the oxygen atom, the electrons are distributed along the energy reserve at two energy levels.
The first energy level, n = 1.
l = 0; s-sublevel; 1s
ml= 0: one s-orbital. ![]()
Second energy level; n = 2; l = 0, 1;
l = 0; s-sublevel; 2s
ml = 0 ⇒ одна × one orbital. ![]()
l = 1; sublevel; 2P
ml = +1, 0, -1 ⇒ три × three orbitals; ![]()
IV. Homework assignment
Describe the state of electrons in an atom of chemical elements № 3, 16, 5, 14, 2; questions 1, 2, 4, 5, 6 (verbally).
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.