
Lesson-seminar on " Electronic structure of the atom»
The purpose of the lesson: to summarize knowledge about the electronic structure of atoms of chemical elements; to consolidate the skills and knowledge of how to write electronic formulae of atoms of chemical elements and their graphic images; to learn the basic concepts of the elementary particles of the microcosm, the twofold nature of the microparticles, electron cloud, atomic orbital, radius, AO, quantum numbers and their characteristics, collection elements, e-formula, a graphical image of the electronic formula; the symmetry of the atom.
Equipment: PSHA of D. I. Mendeleev; the questions of the seminar (theory and practice) on totransparency or in printed form.
Lesson progress
I. Organizational moment
Discussion of theory issues can be organized by working in groups. It should be noted that theory questions include nodal topic questions as well as homework questions. In each group (no more than 5 students) of different levels of academic achievement in the subject, questions are discussed together, and answers are given in oral form. The senior in the group records the work of each student.
The teacher can distribute the theory questions (two at a time) to each group, and then an oral answer is given. Preparing the response can take no more than 5-7 minutes. Discussion of theory questions should not exceed 1.5 minutes.
In the future, the questions of the practical part are completed (it is desirable to complete all tasks in each group). Practice questions will take 10-15 minutes to complete and discuss.
In the remaining 10 minutes of the lesson, the student must perform independent work on options I—II. The teacher regulates the time of discussion of questions and answers. 45 minutes of the lesson is quite enough to solve all the goals and objectives of the workshop.
Theoretical part
1. What models of atomic structure is known?
2. What is the modern model of the structure of the atom?
3. Composition of the nucleus of an atom. Isotopes.
4. Explain the" dual nature " of micro-world particles.
5. define the concepts of "electronic cloud", "atomic orbital", "AO radius".
6. Quantum numbers and their use to characterize the state of an electron in an atom.
7. What principles and rules are known for filling the electron shells of atoms? What would have happened if the Pauli principle had not been followed? Hund's Rule?
8. What e-family is distributed all chemical elements? Why?
9. What is an electronic formula and its graphic representation?
10. What is the" failure " of an electron?
An example of a chemical element that has this phenomenon. What is the reason for the" failure " of the electron?
Practical part
1. What is the composition of atoms of chemical elements?
a) No. 37; b) No. 50; C) No. 74; d) No. 56; e) No. 80.
2. Can such orbitals exist ??
a) 1d; b) 5f; C) 3P; d) 2dd; e) 6d.
Give a reasonable answer.
3. can the electrons of the atoms of the following elements be located in the following orbitals?
a) CA-2P; 1P; 4P; 3d; 3s;
b) Si-4s; 2P; 3P; 2d; 3s;
C) Br-1P; 2d; 2P; 4s; 3d;
d) Mn — 1s; 2P; 2d; 4P; 4s;
e) Sr-6s; 3d; 4d; 4p; 2p.
Give a reasonable answer.
4. Determine which elements are represented by formulas:
a) 3s2; b) 4s23d6; C) 4s24p3; d) 5s24d1, e) 3s23p5.
Give a reasonable answer.
II. Independent work
|
Option I |
Option II |
|
Create electronic and electronic-graphic formulas for the element. |
|
|
№ 56 |
№ 39 |
|
To determine the element which corresponds to the formula. |
|
|
a) 6s1 b) 4s23d2 |
a) 5s24d5 b) 3s23p2 |
|
Give a reasonable answer. |
|
The lesson-seminar ends with summing up the results of students ' work in groups, using the report of the elders in the group, and setting a school grade.
It is advisable to check the workbooks of students who have some problems in studying the subject, as well as the notebooks of students who want to pass them.
It is necessary to analyze the performance of not only the practical part of the seminar, but also the performance of homework.
III. Homework assignment
Repeat § 1-3, notes in notebooks, # 4 § 3.
Answers
Theoretical part
1. The following models of atomic structure:
«ПудингеRaisin pudding" (1902-1904 G. J. Thomson);
"Planetarnaya" (1907 by E. Rutherford);
"The Bohr model" (1913)
2. Modern model of the structure of the atom: an atom is an electroneutral particle; the nucleus of an atom is positively charged and electrons that rotate around the nucleus at a certain speed, having a dual nature, are negatively charged.
3. the Nucleus of an atom consists of protons having mass 1, charge +1, 4 neutrons having mass I and charge 0, the charge of the nucleus is determined by the number of protons. The number of protons corresponds to the ordinal number of the element in The Mendeleev PSE.
Isotopes are a collection of atoms that have the same number of protons in the nucleus of an atom, but differ in the number of neutrons in the nucleus of an atom. The isotopes differ in atomic mass (A). the number of neutrons is determined by the formula N = A - Z.
Z — the sequential number of the element.
4. Particles of the microcosm: electrons, neutrons, protons-have properties that cannot be described by the laws of mechanics for bodies of the macrocosm. The science of quantum mechanics provides an explanation for the dual properties of particles in the microcosm: an electron is a particle that has a certain mass ml= 9 * 10-28, a speedof movement 10.8 cm/sec, a charge -1, and also manifests wave properties; experiments of a group of British scientists in 1927 confirmed the phenomena of diffraction and interoherence.
5. For particles of the microcosm that have dual properties, it is only possible to calculate the probability of finding a particle in a certain volume of space, since wave motion is movement over the entire volume of space occupied by waves, for microparticles there are always uncertainties in the coordinate and momentum, an electron cloud is the space near the nucleus of an atom, where all the mass of an electron and electron density are concentrated. The atomic orbital is a part of an electron cloud where more than 90% of the electron density is concentrated; the AO radius is the distance from the nucleus of an atom to the maximum electron density.
6. Known energy-quantum-numbers that describe the state of an electron in an atom:
n is the main quantum number that characterizes the total energy of an electron of a given energy level, the period number of the PSE corresponds to the number of energy levels in an atom, and it takes integer values;
l — a side quantum number; specifies the energy reserve of an electron at the energy level, characterizes the binding energy of e with the nucleus, and also the form of AO. Accepts values from 0 to n-1;
l = 0-sublevel s, the shape of the orbital is spherical;
l = 1-sublevel p, volume form of the orbital;
l = 2-sublevel d, a more complex form of the orbital;
l = 3-sublevel f is a more complex form of the orbital.
The energy level number corresponds to the number of sublevels at a given energy level.
ml— magnetic orbital quantum number, corresponds to the distribution of AO in the space around the core, determines the number of AO, takes values from -1, 0, +1;
ms-a magnetic spin quantum number characterizes a purely quantum property of an electron; it is the electron's own moment of momentum; the absolute value of spin 1/2, the projection of spin on the axis (magnetic spin number) can have only two values ms= +1/2; ms= -1/2.
7. The principle of minimum energy:
Pauli's principle, Hund's rule , and Kleczkowski'srule .
If the Pauli principle is not observed on AO, the atom would have electrons with the same values of all quantum numbers, i.e., electrons with parallel spins can get into the cells. If the Hund rule is not followed, the total spin will not be the maximum, and this corresponds to a larger value of the atom's energy. This state is considered unstable, which corresponds to the excited state of the atom.
8. Electronic families:
s-elements, if the s-sublevel is filled in;
p-elements, if the p-sublevel is filled in;
d-elements, if the d-sublevel is filled in;
f-elements, if the f-sublevel is filled in.
9. the Electronic formula of an atom of a chemical element shows how the electrons in an atom are distributed, taking into account their characteristic by quantum numbers.
The electronic formula specifies the number of the energy level, sublevels, and the number of electrons on sublevels. The electron-graphic formula shows the quantitiesof AOhidden at energy levels, sublevels in the form of cells, and vectors in the cells are recorded as either unpaired or paired electrons.
10. in the atoms of some elements, the electron passes from the s-sublevel of the external energy level to the d-sublevel of the external energy level. There is a gain in energy. An atom is considered symmetrical, i.e. either most of the electrons become unpaired, or they become paired.
Practical part
1.
|
Chem. the item |
Charge |
||||
|
number |
, the name |
of nucleus |
protons |
neutrons |
electrons |
|
37 |
rubidium |
+ 37 |
37 |
85 - 37 = 38 |
37 |
|
50 |
tin |
+ 50 |
50 |
119 - 50 = 69 |
50 |
|
74 |
tungsten |
+ 74 |
74 |
184 - 74 = 110 |
74 |
|
56 |
barium |
+ 56 |
56 |
137 - 56 = 81 |
56 |
|
80 |
mercury |
+ 80 |
80 |
201 - 80 = 121 |
80 |
2. a) 1d-no, since n = 1, l = 0-открытthe s-sublevel can be opened;
b) 5f-Yes, since n = 5, l = 0, 1, 2, 3, 4 when l = 4-f is the sublevel;
C) 3P-Yes, because n = 3; l = 0, 1, 2 at l = 1, p-sublevel;
d) 2d-no, because n = 2; l = 0, 1 can be opened s-sublevel, p-sublevel;
e) 6d-Yes, because n = 6, l = 0, 1, 2, 3, 4, 5 when l = 4, f is the sublevel.
3. CA — 2p, 1p, 4p, 3d, 3s;
can: 2P, 3s, 4s; no: 1P, 4P, 3d;
Si — 4s, 2p, 3p, 2d, 3s;
2p, 3s, 3p-can; can't-2d, 4s;
In ther — 1p, 2d, 2p, 4s, 3d;
can — 2p, 4s, 3d; no — 1p; 2d;
Mn — 1s, 2p, 3d, 4p, 4s;
can — 1s, 2p, 3d, 4s; no — 4p;
Sr — 6s, 3d, 4d, 4p, 2p;
can — 2p, 3d, 4p; no-4d, 6s.
4. a) 3s2; n = 3 ⇒; element of the III period; 2 e- - II group; - s-sublevel-main subgroup. This is Mg (magnesium).
b) 4s23d6; n = 4 ⇒→ element of the IV period; sum of electrons s + d = 8 ⇒→ element of the VIII group, d-sublevel is filled, element of the side subgroup. This is Fe (iron).
C) 4s24p3; n = 4 ⇒→ element IV of the period; sum of s-and p-electrons 2 + 3 = 5 ⇒→ V the group is filled in, p-sublevel-the main subgroup. This is As (arsenic).
d) 5s24d1; n = 5 ⇒? element of the V period, the sum of electrons s and d 2 + 1 = 3 ⇒? element of the III group, d-sublevel — side subgroup is filled in. This is Y (yttrium).
e) 3s23P5; n = 3 ⇒? element of the III period, the sum of electrons s and p 2 + 5 = 7 ⇒? element of the VII group, filled p-sublevel-the main subgroup. This is Cl (chlorine).
Independent work
Option I
1. No. 56, bariumVA:
the charge of the nucleus is + 56; in the atom 56 e-.
n = 6, six energy levels.
![]()
(the xenon structure is repeated)
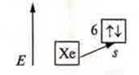
2. a) 6s1; n = 6 ⇒× period IV element, one electron at the external level — group I element;
fill in the s-sublevel and main subgroup.
This is Cs (caesium);
b) 4s23d2; n = 4 ⇒? element of the IV period; the sum of s-and d-electrons 2 + 2 = 4 ⇒? element of the IV group, since the d-sublevel — a side subgroup is filled in. This is Ti (titanium).
Option II
1. No. 39 yttrium Y;
the charge of the nucleus is + 39; in the atom 56 e-.
n = 5, six energy levels.
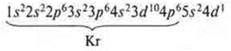
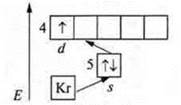
2. a) 5s24d5; n = 5 ⇒× element of the fifth period.
The sum of electrons s and d: 2 + 5 = 7 ⇒? this is the VII group, the d-sublevel is filled ⇒in? the side subgroup. This is TC (technetium).
b) 3s23p2; n = 3 ⇒→ element of the III period; sum of electrons s and p; 2 + 2 = 4 ⇒→ IV group; filled p-sublevel → main subgroup. This is Si (silicon).
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.