
Metals-SUBSTANCES AND their PROPERTIES - LESSON PLANS for CHEMISTRY 11 class - lesson plans-lesson plans-author's lessons-plan-lesson summary - chemistry
The purpose of the lesson: to generalize and consolidate the theoretical knowledge of students about the structure of metal atoms, degrees of oxidation, chemical bonding, and features of physical properties of metals.
Basic concepts: metallicity, electronic family, microelement, microelement, metal bond, polymorphism, allotropy, metal crystal lattice, heavy and light metal, refractory and low-melting metal, electrical conductivity, plasticity, ferromagnetic, paramagnetic and diamagnetic ability.
Equipment: collection "Metals and alloys", models of crystal lattices, table "Types of crystal lattices", tin white and tin gray.
Lesson progress
I. Independent work
|
Option I |
Option II |
|
1. Will these compounds exhibit dual properties? Give a reasonable answer. |
|
|
|
|
|
2. Write down the structural formulas of isomers (two) and homologues (two) of the composition. |
|
|
S5N12O2 |
S5N10About |
|
Give them names and classification characteristics. |
|
Answers to self-study questions
Option I
1.
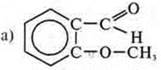 —
this compound is heterofunctional; it shows dual properties.
—
this compound is heterofunctional; it shows dual properties.
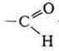 —
aldehyde group. —O is a simple ether group. The compound exhibits the
properties of an aromatic aldehyde and a simple aromatic ether.
—
aldehyde group. —O is a simple ether group. The compound exhibits the
properties of an aromatic aldehyde and a simple aromatic ether.
By classification: cyclic, carbocyclic, aromatic, oxygen-containing.
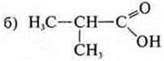 —
monofunctional connection;
—
monofunctional connection;
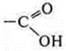 —
carboxylic acid.
—
carboxylic acid.
Acyclic, limiting, oxygen-retaining.
2. C5H10O2CnH2nO2— either an acid or an ester.
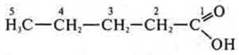 "pentanoic
acid.
"pentanoic
acid.
Acyclic, limiting, oxygen-containing.
Isomers:
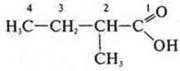 —
2-methylbutanoic acid;
—
2-methylbutanoic acid;
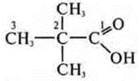 —
2,2-dimethylpropanoic acid.
—
2,2-dimethylpropanoic acid.
Homologues:
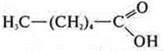 —
hexanoic acid;
—
hexanoic acid;
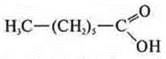 —
heptanoic acid."
—
heptanoic acid."
Ester — inter-class.
Isomers:
![]() —
propyl ether of ethanoic acid;
—
propyl ether of ethanoic acid;
![]() —
ethyl ether of propanoic acid.
—
ethyl ether of propanoic acid.
Homologues (by carboxylic acid):
![]() —
propyl ether of propanoic acid;
—
propyl ether of propanoic acid;
![]() —
propyl ether of butanoic acid.
—
propyl ether of butanoic acid.
Option II
1.
a) H3S-SN = SN-SN3-acyclic, unsaturated, alkene; properties of alkenes;
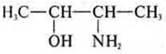 —
acyclic, limiting, oxygen-nitrogen-containing. —ON - alcohol monoatomic — - NH2-amine.
Property of alcohols and amines-heterofunctional, amino-alcohol, dual
properties.
—
acyclic, limiting, oxygen-nitrogen-containing. —ON - alcohol monoatomic — - NH2-amine.
Property of alcohols and amines-heterofunctional, amino-alcohol, dual
properties.
2. With5N10O. CnN2nO is either an aldehyde or a ketone.
Aldehyde:
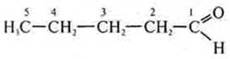 -
pentanal; acyclic, limiting, oxygen-containing.
-
pentanal; acyclic, limiting, oxygen-containing.
Isomers:
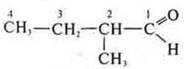 —
2-methylbutanal;
—
2-methylbutanal;
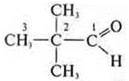 —
2,2-dimethylpropanal;
—
2,2-dimethylpropanal;
Homologues:
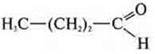 -
butanal;
-
butanal;
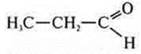 —
propanal;
—
propanal;
Ketone is an interclass isomer:
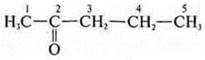 -
pentanone-2;
-
pentanone-2;
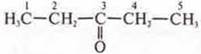 -
pentanone-3;
-
pentanone-3;
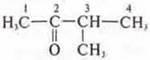 —
3-methylbutane-2;
—
3-methylbutane-2;
Homologues:
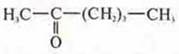 -
hexanon-2;
-
hexanon-2;
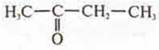 -
butanone-2.
-
butanone-2.
II. Learning new material
Study plan
1. Chemical elements-metals: the position in the Mendeleev PSE; structure of metal atoms; electronic family: S. O. metals based on the position in the system; regularities of metallicity change.
2. Simple substances-metals: chemical bond, crystal lattice, General physical properties of metals, polymorphism.
The teacher focuses the attention of students on the importance of studying this topic and asks them to read the paragraphs of the text of § 18, p. 192, which begin as follows: "Simple substances formed by chemical elements — metals and complex metal-containing substances play a major role in the mineral and organic" life"of the Earth."
After reading these paragraphs, attention is drawn to the concepts of "macronutrient" and "microelement". Students are presented with table 16 p. 193 "the Influence of a lack and excess of metal ions on the state of plants and animals".
Apparently, this exceptional value of metals is due to their unique properties. What properties of metals are known? Students must remember electricalо- and thermal conductivity, plasticity, metallic luster, hardness (except mercury), etc. To explain all this, it is necessary to consistently answer the question of where the metals are located in The Mendeleev PSE.
Answer: If В you mentally draw a diagonal from B to At, then in the lower left corner of the system there are metals, as well as side subgroups of groups V, VI, VII, and VIII.
They are mostly located at the beginning of periods and at the end of groups. Metal atoms are filled with sublevels s -, p-, d -, f -, so they form similar families; s-and p — families are the metals of the main subgroups, d - and f - families are the metals of the side subgroups.
The vast majority of elements in the system are metal. At the external energy level, they have 1-2 electrons, less often 3-4. Metal atoms are characterized by a small electronegativity, they have only reducing properties — to give up electrons. The reduction capacity of metals increases by the beginning of the period and by the end of the main subgroup, because the atomic radius increases.
For metals of side groups, the radius of the atom changes slightly with increasing nuclear charge, because the pre-external energy level is filled. Therefore, the bond strength of valence electrons with the nucleus increases, and the reducing properties weaken.
Thus, the charge of the nucleus of an atom, the radius of an atom affect the reducing properties of all metals.
C. O. of metals is determined by valence electrons of external and external energy levels:
For s-and p-elements — the number of s electrons or the sum of s-and p-electrons:
Na +1; Mg +2; Al +3.
The d elements of S. O is determined by the valence electrons outer energy level is the minimal S. O., and the sum of s-electrons of the outer energy level and d-electrons predvneshnem energy level is a maximum C. O., it is, respectively, equal to the group number.
d-elements can also exhibit intermediate CPS, which can give stable compounds.
Example: Mn.
Minimum CPR = +2
Maximum CPR = +7
Intermediate CA: +4, +6
All metals of simple substances are characterized by common physical properties, which are due to the fact that all metals have a metal bond and the type of lattice is metallic.
Let's remember what a metal bond is, what it is caused by. Let's look at the drawings of a metal bond in the table and in the textbook on page 196, and also read the definition of this bond.
A metal bond is a bond in metals and alloys between metal-ion atoms located in the nodes of a crystal lattice, which is carried out by socialized electrons.
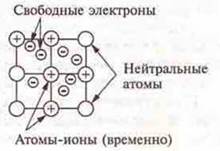
The features of metal bonding are due to the following. A small number of electrons simultaneously penetrate many atomic nuclei, this bond is non-localized, the electrons move freely throughout the metal crystal, they are called free; the saturation and direction of the metal bond does not possess.
When the temperature changes, there is a change in the amplitude of oscillation of ions-atoms in the crystal, which interferes with the directed movement of electrons, and the electrical conductivity changes.
When t° is lowered, the electrical conductivity increases; at absolute zero, most metals have superconductivity. The best metals in terms of electrical conductivity are Au, Ag, Cu,Al; less electrically conductive are Hg, Pb, MP.
Thermal conductivity changes with the same regularity. Free electrons collide with oscillating ions-atoms, exchange energy with them, as a result of which there is an equalization of temperature on the piece of metal. In all metals, such indicators as mechanical strength, melting and boiling points, and density differ greatly.
Example:
![]()
The reason for this difference is that metals form different forms of the crystal lattice.
Metals are characterized by a metallic crystal lattice of several forms:
a) cubic face-centered (Withu, Ni, Fe);
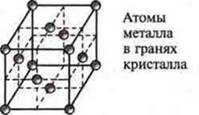
b) cubic volume-centered (V, Nb, W, Cr, Na, etc.);
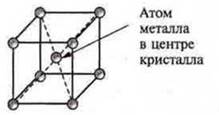
C) hexagonal (Mg, Ti, Sr, Zn). P. 34 (II CH.)
According to this structure all metals have common physical properties:
1. Plasticity — displacement of metal ion layers under external influence on the crystal relative to each other; since free electrons move throughout the crystal, the bond breakage does not occur; ionic crystal lattices are destroyed). In the textbook p. 198, Fig. 40.
2. Metallic luster (presence of free electrons). As a comparison, we note the metallic luster уof solid iodine-silicon. This indicates the appearance of a certain metallicity of some non-metals.
Free electrons reflect light rays, and do not pass through like glass (depending on the degree of scattering, all the rays of the sawn part of the spectrum). Metals are therefore silvery-white or gray in color. The exceptions are Sr, Au,And u-they absorb short rays and reflect long waves of the light spectrum and therefore have colors: Sr-light yellow, Au-yellow, Withu — "copper color", red. If the metal is in the form of a powder, then it does not always have a metallic luster — black or gray; if the metal is in the form of a thin foil, then it can be, for example, (silver-blue color, Golden-green color.
3.the Electricaland thermal conductivity of metals is due to the fact that chaotically moving electrons in a crystal acquire a directed movement under the action of voltage. An electric current is the directed movement of charged particles.
Some metals, depending on the conditions, can crystallize in two or more crystalline forms. This phenomenon is called polymorphism (allotropy).
Example:
Tin-white tin-β-stable at t° above 13.2°. Grey tin-α; stable at t° below 13.2°.
Iron has four crystalline modifications: α, β, γ, δ. Under what conditions is specified in the textbook p. 196-197.
Metals also differ in their relation to the magnetic field:
ferromagnetic metals-magnetized (Ni, Fe (α));
paramagnetic metals-weakly magnetized (Al, Cr);
metals are diamagnetic — they are not attracted to the magnet; they are repelled (Sn, Withu).
D-elements are characterized by the formation of covalent bonds (W, Mo, Cr, Os), so they are refractory, hard and heavy.
Many values of density, boiling point, and melting point are difficult to remember. It is desirable to know the following classification of metals by physical properties.
|
Electrical conductivity |
High |
(Ag, 6 * 107ohms-1· m-1) |
|
Low |
(Mn, 5 · 105ohms-1· m-1) |
|
|
Melting |
point low Melting |
point t°PL< 1000° With |
|
Refractory |
t°PL> 100>° With |
|
|
Density |
Light density |
ρ < 5 g / cm33 |
|
Heavy |
ρ > 5 g/cm>3 |
|
|
Hardness |
Soft |
They are cut with a knife |
|
Hard |
Scratch glass |
According to the degree of proximity of physical and chemical properties, metal groups are distinguished: alkaline, alkaline earth.
III. Homework assignment
§ 18 to p. 201; no. 1, no. 2.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.