
Genetic connection of organic and inorganic compounds-SUBSTANCES AND their PROPERTIES - LESSON DEVELOPMENTS in CHEMISTRY 11 class - lesson developments-lesson developments-author's lessons-plan-lesson summary - chemistry
The purpose of the lesson: to consolidate the knowledge of the concepts of "genetic series" and "genetic connection"; to teach to characterize the relationship between the main classes of substances in organic and inorganic chemistry and correctly draw up schemes of transformations and solve them; to give an idea of the unity of organic and inorganic substances, about their relationship.
Basic concepts: genetic series, genetic connection, transformation scheme.
Equipment: reagent kit, alcohol lamp, test tube holder, test tubes; Ag2O(NH3· H2O); spoon for burning substances; KMnO4, H2SO4(K), water, Fe, exhaust pipe.
Lesson progress
I. Organizational moment
Setting the goals and objectives of the lesson and analyzing the results of practical work # 4. inform students That this lesson is a preparation lesson for practical work # 5, which will take place in the next lesson.
II. Learning new material
Plan of presentation
1. a) the Concept of "genetic series" in inorganic and organic chemistry.
b) Genetic range of metal and nonmetal in inorganic chemistry.
C) Genetic series of methane, ethane, propane in organic chemistry.
d) Experimental implementation of the genetic series of copper and sulfur, according to the schemes:
![]()
2. Genetic relationship between classes of inorganic compounds. Experimental implementation of a genetic link according to the scheme:
![]()
3. Genetic connection between classes of organic compounds (textbook, p. 266).
Pilot implementation of the transformation scheme:
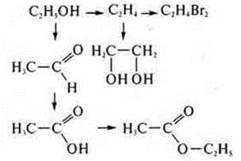
4. Interrelation of organic and inorganic chemistry substances.
The genetic series includes substances formed by the same element, but belonging to different classes, that is, they reflect different forms of its existence.
In inorganic chemistry, the genetic series of a metal and the genetic series of a non-metal are considered.
Let us consider the genetic series of copper as an example and implement it experimentally.
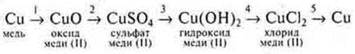
There are five transformations in this row.
![]() - we
calcinate the copper wire, it becomes covered with a black coating — this is
copper (II) oxide.
- we
calcinate the copper wire, it becomes covered with a black coating — this is
copper (II) oxide.
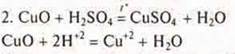
Add sulfuric acid to the resulting copper (II) oxide and heat it up. There is a change in the color of the solution, it becomes blue. Hydrated copper cations are formed, the copper salt — copper (II) sulfate.
3.Add sodium hydroxyl to the CuSO 4 saltuntil the blue-colored precipitate - copper (II) base hydroxyl is insoluble:
![]()
4. Filter out Cu(OH)2 and add HCl-hydrochloric acid to it:
![]() — the
sediment disappears.
— the
sediment disappears.
![]()
5. To a solution СuСl2— copper chloride (II) added Fe — iron. Copper formation is observed.
![]()
The genetic series of a nonmetal is considered by the example of a nonmetal S-sulfur.
![]()
1. Burning sulphur in a spoon for burning substances. Sulfur burns with a blue flame in a flask of oxygen (oxygen was obtained in advance by decomposition of Cmpo4and filled the flask with it)
![]()
The flask is filled with gas.
2. in this flask, add distilled water with a few drops of the methylorange indicator and shake thoroughly. There is an interaction of SO2with H2O, the indicator turns pink, which indicates the presence of acid.
![]()
3. To a small quantity of sulphurous acid in a test tube add sodium hydroxide until the. until themethylorange becomes orange.
![]()
4-5. add hydrochloric acid to the sodium sulfite — there is a release of so2 gas, which must be sent through the gas outlet tube to a test tube with a solution of hydrogen sulfide; there is a turbidity of the solution, sulfur is formed.
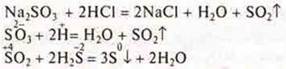
Genetic series of organic compounds: methane, ethane, propane in the form of transformation schemes, the teacher writes on the blackboard, students-in notebooks.
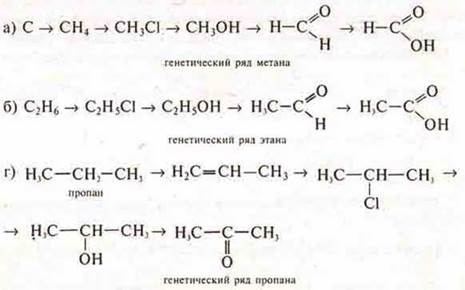
However, the transformation schemes may include substances of a different composition. Here we are already talking about the genetic connection of substances, which is realized in any mutual transformations of substances with different compositions and belonging to different classes in both inorganic and organic chemistry.
Experimentally, we will perform a chain of transformations that includes substances of different classes and different compositions.
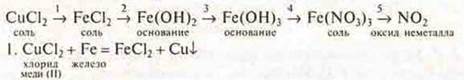
![]() —
filter out copper, and2 add NaOH tothe FeCl 2 filtrate .
—
filter out copper, and2 add NaOH tothe FeCl 2 filtrate .
![]()
3. In the air in the presence of oxen Fe(OH)2 it is oxidized by oxygen.
![]()
4. To the draught of Fe(OH)3add nitric acid:
![]() —
iron nitrate (III)
—
iron nitrate (III)
![]()
5. When heated small amounts of the salt solution is observed the allocation of the brown gas NO2(nitrogen oxide (IV)):
![]()
In the textbook on page 266, more voluminous schemes of the genetic connection of organic compounds are given. On the basis of these schemes, you can make any scheme of transformations from an alkane to a polypeptide protein. Experimentally, we will implement a transformation scheme:
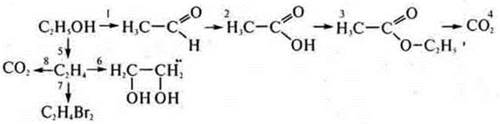
1. in a test tube with ethanol, insert a calcined copper wire up to 10 times.
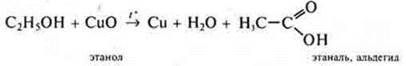
2. We conduct a reaction with copper (II) hydroxide when heated (in the absence of the reagent Ag2O(NH3· H2O), which gives the "silver mirror"reaction). Getting Fromu(ON)2:
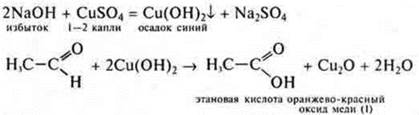
3. add ethanol To acetic acid (in the same amounts) and 1-2 drops of H2SO4(conc.), heat the mixture.
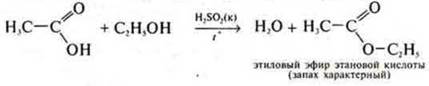
4. at the entrance of the exhaust pipe, we ignite the ether.
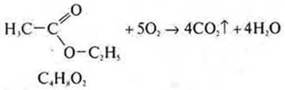
5. Add H 2 SO 4(K2SO); a ratio of 1 : 3 is required.
Vethanol= 1 ml; VH2SO4= 3 ml. This is an intramolecular dehydration reaction, occurring at t° > 140°:
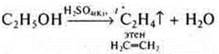
6—7. The resulting ethene is passed through solutions of KMPO4and I2; there is a discoloration of the solutions; at the exit of the gas outlet tube, ethene is ignited. It burns with a yellow, glowing flame

It is possible to consider the reaction as an IAB in an explicit form.
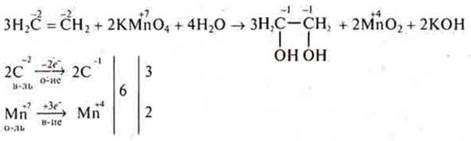
R-R Yoda is discolored.
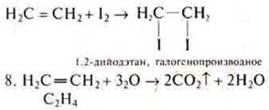
At the beginning of the nineteenth century, the prevailing view was that there was a clear line between inorganic and organic substances. But the first syntheses of organic substances showed the inconsistency of these views.
German chemist F. Wehler synthesized oxalic acid from inorganic compounds:
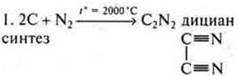
2. Hydrolysis Tiziana:
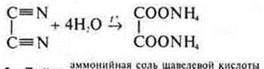
3. By the action of an inorganic acid on this salt oxalic acid is obtained:
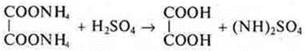
In 1928 F. Wohler carried out the synthesis of urea.
Urea: - CO(NH2) - a product of the vital activity of living organisms. In the laboratory, he synthesized an inorganic substance-цианатammonium cyanate:
![]()
Then he heated it in an aqueous solution and got urea:
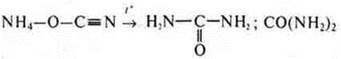
All these syntheses prove that organic substances can also be formed from inorganicsubstances, bypassing the living organism. These are the first syntheses, followed by many others that confirmed the connection between inorganic and organic substances.
III. Homework assignment
§ 23: 1. Schemes of transformations: option 1-no. 1) g. no. 2) b; option 2-no. 1) d, no. 2) C; 2. Make a scheme of transformations and solve it:
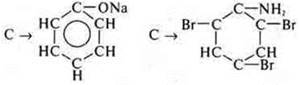
Do the work on separate sheets, for evaluation.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.