
Laboratory work№2
Topic: Qualitative reactions to organic compounds
The purpose of the work: to get acquainted with the methods of discovering organic substances and their main elements.
Necessary equipment: some organic substance (aniline, its chlorhydrates, thiomochevine, iodoform, sulfanylic acid), metal sodium, lead nitrate, sodium nitroprussid, iron sulfate (II), chloroform or benzene.
№1-experience. Determination of sulfur, nitrogen, and halides in organic compounds.
Course of work: we put a few crystals or drops of our detecting substance in a test tube. Place the test tube obliquely and place a metal sodium from wheat grains, peeled from the shell, dried on filter paper, on top.
We heat the test tube in the horizontal direction first until the sodium melts, and then hold it in the vertical direction until hot drops of sodium get into the detecting substance. The mixture is strongly heated, put in a bowl with 5-6 ml of water and break the test tube. This practice is performed in a rack cabinet.
We grind the black particles of the mold, pour the liquid into a test tube and heat it until it boils. By filtering the alkaline liquid, we observe the effect on sulfur and nitrogen on halides. The liquid should be colorless.
a) determination of sulfur:
1.1 ml of lead nitrate is dripped with sodium hydroxide until the first formed precipitate dissolves. Then we add a few alkaline liquids. The appearance of a dark brown color or black precipitate of lead sulfide indicates the presence of sulfur in our detected substance.
2.add 1-2 drops of sodium nitroprussid to 1 ml of alkaline liquid. If there is sulfur, then the mixture quickly or gradually gives a bright purple color.
B) determination of nitrogen:
In the alkaline liquid, we put a small crystal of iron cooperos, boil for 1-2 minutes, cool for 3-5 minutes and add liquefied hydrochloric acid. The appearance of blue tincture of Berlin Azure indicates the presence of nitrogen.
C) determination of halides:
Acidify the alkaline liquid with nitric acid. Boil for a few minutes in a pull-out cabinet. Allow to cool, add a few drops of silver nitrate. The appearance of a heavy cotton precipitate indicates the presence of halogen. AgCl-White, MeBr - yellow, MeJ - yellow. To determine if there is Br or J, we add 1 ml of chloroform and add 1-2 drops of KMnO4 with a shake. Purple indicates that there is a color - J, red indicates that there is a yellow-Br.
№2-experience. Determination of organic compounds carbon and hydrogen.
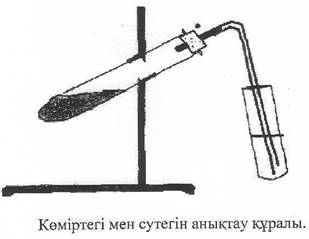
Course of work: 0.2-0.3 g of the determining substance in glass is treated with 1 - 2 g of copper oxide (powder). The mixture is placed in a dry test tube and firmly fixed with a cork with a gas-conducting tube. We attach the test tube to the tripod in the horizontal direction. We insert the end of the tube into a test tube with 2-3 ml of colorless lime or barite water. The mixture is first simply heated more strongly after. We see that the droplets on the cool walls of the test tube and tube form water and form carbon dioxide, calcium or barium from the formation of a carbon dioxide precipitate.
№3-experience. The discovery of halides is the Beylstein reaction.
Working process: for a thin red copper wire, we make a loop with a diameter of 1-2 and heat it up. We cool the blackened Wire, take the object we identify with the hanger and hold it on the flame.
If there is a halogen, then the flame will give a green color. To clean the wire, we soak the hydrochloric acid and reheat it.
Control questions:
1. What is the simplest way to distinguish an organic substance from an inorganic one?
2. How are the elements in organic compounds qualitatively determined?
3. What reaction occurred in barium hydroxide?
4. What is the basis for the presence of carbon and hydrogen in organic matter, write a summary?
5. What is the essence of the Beylstein sample?
6. What are the other ways to discover nitrogen in organic compounds?
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.