
Instruction card
Topic: Adiabatic process, Poisson’s equation
Learning objectives:
10.3.3.2 – apply the first law of thermodynamics to isoprocesses and adiabatic processes
Write a plan of your work for this lesson:
Task 2.
The table below describes two types of adiabatic process.
|
Process name |
Adiabatic expansion |
Adiabatic compression |
|
Graph of the process
|
|
|
|
Mathematical equation |
|
|
|
Change of internal energy |
ΔU<0 U↓ T↓ |
ΔU>0 U↑ T↑ |
|
Work done by the system |
Q=0 A' > 0 ΔU=-A' < 0 A'=-ΔU |
Q=0 A>0 ΔU=A |
|
Process description |
The system performs mechanical work only by reducing its internal energy. |
The internal energy of the system increases due to the work of external forces. |
Using the table,
(а) Determine whether they change or remain constant.
(i) pressure ___________________________
(ii) volume _____________________________
(iii) temperature _______________________
(b) Determine whether the adiabatic process is an isoprocess. Explain your answer.
__________________________________________________________
(с) below the line contains the values included in the first law of thermodynamics. Underline which one is always zero.
|
Quantity of heat |
Change in internal energy |
Work done (on/by) |
(d) Select in the table the mathematical record of the first law of thermodynamics for the adiabatic process.
(е) Try to formulate the definition of an adiabatic process.
__________________________________________________________
Work with the source:
http://ru.solverbook.com/spravochnik/uravneniya-po-fizike/uravnenie-puassona/
The task. Read the text.
Write in a notebook:
1) Determination of the adiabatic process
2) Poisson equation
3) Draw an adiabatic plot versus an isotherm.
4) Write down the conditions under which real processes can be considered adiabatic.
The execution of tasks.
1. The figure below shows the graphs of the two processes adiabatic and isothermal. Sign on the picture the name of each process.
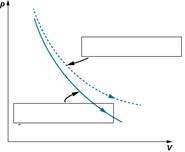 |
2. With adiabatic compression of the gas, 200J was performed. How and how much has the internal energy of a gas changed?
3. In the process of adiabatic expansion, the gas did work equal to 3 · 103J. What is the change in the internal energy of the gas?
4. The monatomic ideal gas, taken in the amount of two moles, expanded without heat exchange with the environment. The gas temperature during expansion decreased by 10 ° C. Determine the work done by gas.
5. Monatomic ideal gas is adiabatically compressed so that its volume has increased by 2 times. How will the gas pressure change?
6. 100 moles of a diatomic ideal gas were adiabatically compressed at 300 K. At the same time, the gas pressure increased by 3 times. What work did the external forces do on the gas?
Скачано с www.znanio.ru
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.