
Amin of aliphatic range
The plan:
1. Homologous amine series.Classification.Isomers . Nomenclature.
2. Physical and chemical properties of amines
3. Extraction and use of amines.
Amines are considered to be compounds substituted for alkyl (R-) groups of hydrogen atoms in ammonia. In the primary, secondary and tertiary amines 1,2,3 hydrogen atoms are converted into radicals.
|
|
||
|
primary amine methylamine |
Secondary amine methylethylamine |
tertiary amine dimethylethylamine |
The isomerism of amines depends on the location of the amine group in the carbon chain, their number and the structure of the nitrogen atom and the bound radicals.
|
|
|||
|
Aliphat amin methylamine |
Acyclic amine cyclohexylamine |
Aromatic amine aniline |
mixed amine N, N-dimethylamin |
According to the system nomenclature, amines are named by adding the amine suffix to the name of the radical.
Physical and chemical properties
Simple amines - methylamine, dimethylamine, trimethylamine gases, soluble in water, smell like ammonia. Other lower amines are ammonia-scented liquids. Complex amines are unpleasant (unpleasant) fish-scented liquids.
High amines are solid, water-insoluble, odorless substances.
Its chemical properties are similar to ammonia.
1. Aqueous solutions of amines show the basic medium:
R- NН2 + НОН « [RNН3]+ + OН-
2. Amines react with mineral acids to form ammonium salts
R-NН2 + НСl → R – NН3+Сl- – converted to alkyl
The basic properties of amines are higher than ammonia.
3. Primary and secondary amines also show weak acidic properties, forming salts-alkyl or dialkylamides with alkali metals:
2R2NН + 2 Nа → 2R2N – Nа + Н2
4Amines are alkylated: primary and secondary amines convert hydrogen of the amine group into alkyls, through which the reaction produces secondary and tertiary amines:
RNН2 + СН3І → R – NН – СН3 + НІ, or R–NНСН3 . НІ
RNНСН3 + СН3І → RN(СН3)2 . НІ
5 Amines can be acylated by reacting with acetic anhydride or acetyl chloride:
С2Н5-NН2 + (СН3-СО)2О → С2Н5-NН-СО-СН3 + СН3СООН
Ethyl acetamide or acetyl methylamine
6. Nitric acid forms primary amines, nitrogen and alcohols, alkenes, esters, secondary amines - nitrosamines
RNН2 + НОNО → R – ОН + N2 + Н2О,
R2NН + НОNО → R2N – NО + Н2О
7. Primary amines are characterized by an isonitrile reaction: in the presence of caustic potash (KOH) primary amines form isocyanides with chloroform, have a very bad odor
R –NН2 + СНСl3 + 3КОН → RNС + 3КСl + 3Н2О
Secondary and tertiary amines do not give these reactions, so they are used to reveal primary amines.
Production and use of amines
1. When alcohol and ammonia vapors are passed through a catalyst (AI2O3; ThO2) at a temperature of 3000C, a mixture of amines (primary, secondary, tertiary) is obtained:
![]()
2. When ammonia reacts with halogen derivatives, a mixture of salts of different amines is obtained.
![]()
chloro methylamine methylamine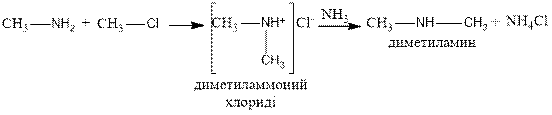



3. Acid amides lose their effect on hypobromite or hypochlorite and give primary amines ( Hoffman reaction):
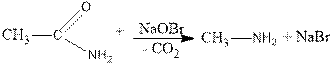
4. In the presence of catalysts (Pt, Pd, Ni) nitro compounds are reduced:
0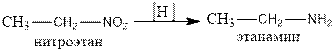
5. Redoxes or catalysts in the presence of Pt, Pd, Ni form primary amines with hydrogen and nitriles.
![]() ,
,
and isocyanides provide secondary amines:
R- NС + 2Н2 → R – NН – СН3
Application:
Amines are used as organic bases and in amination reactions. Amines from sulfuric acid solutions are used to produce uranium. Amines, such as the smell of fish, are used to fight steppe rodents.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.