
Methodological guide
Beginning of the lesson
|
Process |
Name of the Process |
Information about this process |
|
Increase the volume of the balloon when transferring from a cold to a warm room. |
Isobaric |
P= const
|
|
Very slow balloon inflation by bicycle pump. |
Isothermic |
T= const
|
|
Heating an empty glass jar with a tightly closed stopper. |
Isochoric |
V= const
|
For the teacher! It is recommended at this stage to apply self-assessment of their work on the model in pairs.
The teacher can count the number of correct structural units (20), and students will also determine how many of them they recorded correctly.
Next, ask the students to raise their hands, who correctly completed the entire task, made one mistake, and so on.
Further, the teacher informs students that today they will learn to apply the first law of thermodynamics to isoprocesses. He discusses with the students the topic of the lesson, the purpose of the training, the objectives of the lesson and jointly draws up the criteria for evaluation.
Further, they jointly draw up a work plan.
1. Perform an analysis of the isoprocess schedule by the algorithm.
2. Independently apply this algorithm to other isoprocesses.
3. Solve problems with quantitative and graphical data.
Tasks 2 and 3
For the teacher! Students will work in pairs. The first student in the pair analyzes the schedule for the isobaric process, the second - for the isothermal. Each template has a blank second column, which they must fill in during execution.
After the individual assignment, the students are combined into two groups according to the type of the analyzed chart and check the correctness of the implementation. Further, they return in pairs and explain the completed task to each other.
Individual student work
Check in groups
Mutual learning
The teacher draws attention that all the processes shown can proceed in the opposite direction, and together with the students summarizes the material on the table.
Source: http://www.eduspb.com/node/1748 The table in the .doc format is in the applications for the lesson.
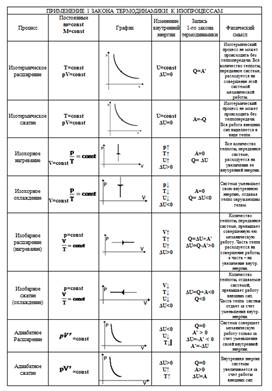 |
Using the table, complete the tasks.
A source: https://nsportal.ru/shkola/fizika/library/2012/04/03/primenenie-pervogo-zakona-termodinamiki
For the teacher! The number of tasks is proposed taking into account the different levels of students' preparedness, it is assumed that 1-3 tasks can be performed orally by most students and will not cause difficulties.
1. When isochoric heating gas was transferred from the heater the amount of heat 250J. What kind of work did gas do? What is the change in internal energy?
Explanation:
Since the process is isochoric, the gas operation is zero. Then, according to the first law of thermodynamics for an isochoric process, the amount of heat transferred to the system will go to change its internal energy.
The answer is: 250J
2. During isobaric heating, the gas received from the surrounding bodies 750 J of heat, while the internal energy of the gas decreased by 250 J. What kind of work did gas do?
Explanation:
In the isobaric process, the amount of heat received is spent on doing work and changing the internal energy. As the internal energy of the gas decreased, he completed work 250 J more than received heat from the surrounding bodies.
Answer: 1000 J
3. When isothermally compressed, the gas transferred the surrounding heat to 800J. What kind of work did the gas do? What work did the external forces do?
Explanation:
In an isothermal process, the amount of heat received is used only for work and vice versa. Since he transferred heat to the surrounding bodies, his volume decreased, respectively, the work would be negative and equal to 800J, then the surrounding bodies performed work on him 800 J.
Answer: -800 J, 800 J
4. One mole of monatomic ideal gas is in a closed vessel at a temperature of 27 °C. How much heat is needed to communicate gas to increase its pressure three times?
Solution:
We take into account that the gas is monatomic and the vessel is closed, and nothing is said about changing its volume, so the process can be considered isochoric.
As the pressure increased threefold, the absolute temperature also increased threefold. Then the temperature change
![]()
Let us apply the first law of thermodynamics to the isochoric process.
![]() we get 7,5 kJ
we get 7,5 kJ
Answer: 7,5 kJ
5. Calculate how much heat was received by hydrogen weighing 2 kg with isochoric heating at 10 K.
Solution:
We take
into account the conclusions made in task 4 and the fact that hydrogen is a
diatomic gas, the amount of matter of which can be calculated by the formula ![]()
![]() we get 208 kJ
we get 208 kJ
Answer: 208 kJ
6. For isobaric heating of the gas, the amount of substance of which is 400 moles, at 300 K he was informed about the amount of heat of 5.4 MJ. Determine the operation of the gas and the increment of its internal energy.
Solution:
Consider that the process isobaric. In this case, Q = ΔU + A. Since we do not know the type of gas, we can find a job using the formula for calculating the work with the isobaric process and the equation of state of an ideal gas.
![]() , we get 9.97
× 105 J
, we get 9.97
× 105 J
From the first law of thermodynamics ΔU = Q-A, we get 44 × 106 J
Answer: А´ = 9.972 · 105 J; ∆U = 4.4 · 106 J
Скачано с www.znanio.ru
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.