
Periodic law and Periodic system of chemical elements of D. I. Mendeleev in the light of the doctrine of the structure of the atom
The purpose of the lesson: to give an idea of attempts to classify chemical elements and their consistency, which Were developed by D. I. Mendeleev's predecessors, as well as basic information about the discovery of the law by D. I. Mendeleev; to teach to give a formulation of the law according to Mendeleev, as well as modern ones based on the doctrine of the structure of the atom; to consolidate knowledge of the structure of the periodic system; the physical meaning of the period number, group number, ordinal number.
Basic concepts: Periodic law, Periodic system, isotopes, chemical element, physical meaning of ordinal number, period number, group number; structure of the periodic system, periodicity vertical, diagonal, horizontal, "star".
Equipment: Mendeleev PSCE, portraits of D. I. Mendeleev in different years; portraits of "chemists-predecessors".
Lesson progress
I. Checking the completion of homework
The answers to the questions of § 4
No. 1. the Valence capabilities of chemical element atoms are determined by the number of unpaired electrons, the presence of free orbitals, and the presence неподеленныхof unpaired electron pairs in the atom.
No. 2. at the second energy level, it is possible to open four orbitals: 2s — one orbital and 2P-three orbitals.
# 3. Valence — the number of common electron pairs that an atom forms with other atoms.
No. 4. S. O. — a conditional charge that an atom acquires in a compound, if it is considered that an ionic bond is connectedto an ionicbond.
In both cases, electrons are involved.
№ 5
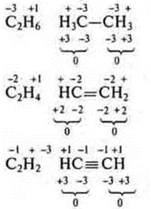
№ 6.
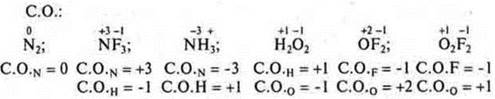
Valence:
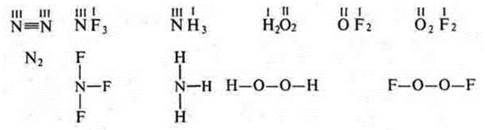
№ 17. Sulfur (S): + 16, 16e-.
![]()
Valence capabilities of a sulfur atom in the ground state.
The valence is two, since there are two unpaired electrons; the donor atom may have a valence of 3 and 4.
Valence possibilities of the sulfur atom in the excited state.
First excited state
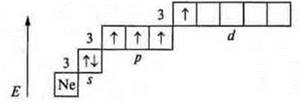
The valence is four, just like the donor atom is five.
The second excited state
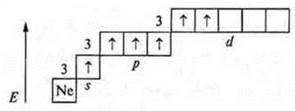
The valency is six.
I. Independent work
|
Option I |
Option II |
|
1. Compare the valence capabilities |
|
|
of oxygen and sulfur atoms |
of nitrogen and phosphorus |
|
in the ground and excited States. Whether they are the same. Give a reasonable answer. |
|
|
2. Determine the valence and CP of elements in compounds |
|
|
|
|
|
3. Determine the Maximum number of elements in a compound |
|
|
|
|
II. Learning new material
Plan of presentation
1. Introduction to the study of this issue (the word of the teacher). D. I. Mendeleev's periodic law and Periodic system of chemical elements are the basis of modern chemistry. They relate to such scientific regularities that reflect phenomena that actually exist in nature and therefore will never lose their meaning (textbook, p. 26 § 5).
While studying this question, think about the following: many scientists were searching for a natural classification of chemical elements, were in equal conditions, had the same prerequisites (see the textbook, pp. 26-29). Why did none of them, except D. I. Mendeleev, manage to discover the Periodic law?
Then there are messages from students on certain issues and a teacher's story.
If there are no messages, then students can work with the textbook at the time of the teacher's story in the same sequence as the students ' messages were supposed to be.
2. student Messages (or a teacher's story, working with the text of the textbook on leading questions according to the topics of messages).
If student messages are provided, then the plan is as follows:
Message 1
Prerequisite for the discovery of D. I. Mendeleev's Periodic law. Congress of chemists in Karlsruhe.
This report reveals the work of scientists who were D. I. Mendeleev's predecessors:
— the classification of Berzelius;
- triadsThe döbereiner (1816);
- the Chancourtois spiral Шанкуртуа(1862);
- octavesNew landsen (1865);
- Meyer's table (1864).
The failure of attempts at classification is explained, and students are also introduced to the important significance in the opening of the Periodic law of the Congress of chemists in Karlsruhe in 1860.
Message 2
D. I. Mendeleev's discovery of the Periodic law.
In this message:
1) acquaintance with the personal qualities of the Russian scientist D. I. Mendeleev, his life, creative heritage;
2) the main points on which D. I. Mendeleev relied in his discovery;
3) structure of the Periodic system proposed by D. I. Mendeleev: natural groups, periods;
4) change in the properties of chemical elements, simple substances, as well as complex compounds or formed in periods-horizontal periodicity:
5) the Periodic law formulated by D. I. Mendeleev on March I, 1869 (textbook, p. 32).
Then the teacher can explain the periodic patterns: vertical (in groups) and diagonal.
Best of all, the diagonal periodicity of the properties of non-metals is characterized by the diagonal B-Si—As-Te—At, which conditionally divides elements into metals and non-metals (or the diagonal C— P—Se—I). The two diagonals AI-Le-Sb and Zn-Tn-Pb include elements and their compounds with amphoteric properties.
Vertical periodicity shows changes in the properties of elements and compounds formed by them in groups or major subgroups. With the growth of the ordinal number, increasing metallicity is the main character of compounds, non-metallicity weakens неметалличность, the strength of volatile hydrogen compounds decreases, and their acidity increases.
If you combine the horizontal vertical and diagonal periodicity, you can get a "star periodicity". The ego allowed D. I. Mendeleev not only to predict and describe the properties of substances formed by yet undiscovered chemical elements, but also to indicate the ways of their discovery, natural sources from which the corresponding simple substances could be obtained.
Message 3
The story of the predictions of the elements No. 21 — acabar, 31 — aqualumiere Mendeleev and opening them.
However, new scientific discoveries began to contradict the formulation of the Periodic law according to D. I. Mendeleev: the discovery of isotopes made it possible to consider as chemical elements the types of atoms characterized by the same charges of the nucleus, i.e. containing the same number of protons.
Isotopes are known in all chemical elements. In nature, most of them exist as a mixture of isotopes. The relative atomic mass of an element is equal to the average value of the relative atomic mass of all its natural isotopes, taking into account their distribution.
Task.
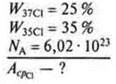
1) N — the number of atoms of the isotope mixture.
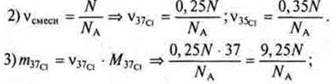
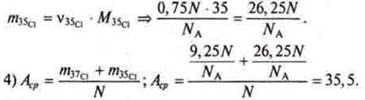
In the Periodic table, under the symbols of chemical elements, the average values of their relative masses are given.
It should be concluded that the presence of isotopes proves that the properties of chemical elements are determined not so much by their atomic mass, as suggested by D. I. Mendeleev, but by the charge of their atomic masses.
This explains the position of four pairs of elements in the system, placed in violation of the principle of increasing atomic masses.
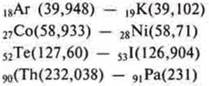
The genius of D. I. Mendeleev, the manifestation of his scientific intuition consists in the fact that he preferred to arrange by similarity in properties, thereby predicting the true order of placement of chemical elements by increasing the charges of their atomic nuclei, although he did not know anything about the structure of their atoms. Theoretically, van de Broek (Holland) and experimentally, the city of London.Moseley (Germany) proved that the charge of the nucleus of an atom corresponds to its ordinal number.
Thus, the discovery of isotopes and the correspondence of the nuclear charge to the ordinal number of a chemical element allowed us to give a modern definition of the Periodic law.
Properties of chemical elements and substances formed by them are in periodic dependence on the charges of their atomic nuclei.
III. Consolidation and synthesis of the studied material on key issues
1. Initial provisions, which were based on the discovery of the law by D. I. Mendeleev.
2. Formulation of the Periodic law by D. I. Mendeleev.
3. The frequency of vertical, horizontal, diagonal, "star".
4. Isotopes. Modern formulation of the Periodic law.
5. The structure of periodic system:
a) periods;
b) groups.
6. The physical meaning of:
a) serial number;
b) period numbers;
C) group numbers in the light of the structure of the atom. Explain using the example of elements # 18, 101, and 74.
IV. Homework assignment
§ 5 before the section "Preparation for chemical dictation".
Answers to self-study questions
Option I
1. Oxygen
![]()
Ground state
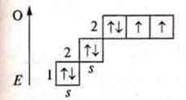
Sulfur
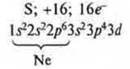
Ground state
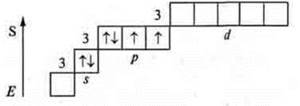
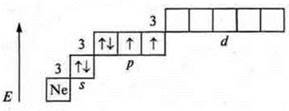
Atoms on the external energy level have two unpaired electrons and there are two pairs of paired electrons, these are donor atoms, the valence possibilities are the same — two, three, four. The oxygen atom cannot have an excited state, because at the second energy level there is no possibility of opening the sublevel d, and the steaming of electrons at 3s requires a huge expenditure E, which is impossible.
The sulfur atom can have an excited state, because at the third energy level, the d-sublevel opens.
Valence four and five, like a donor atom.
Valency six.
Option II
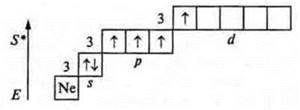
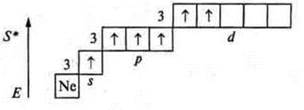
1. Nitrogen
![]()
Phosphorus
![]()
Atoms in the ground state have three unpaired p-electrons and a pair of s-paired electrons at the external energy level.
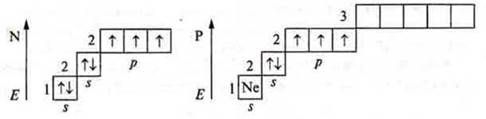
They can manifest valences of three and four, like donor atoms.
The nitrogen atom cannot have an excited state, because there is no sublevel d at the second energy level; the transition to the third energy level requires a huge expenditure of energy, which is impossible. At the phosphorus atom, the excited state is possible, since the 3d sublevel opens.
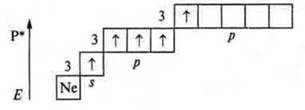
The valence of the phosphorus atom is 5.
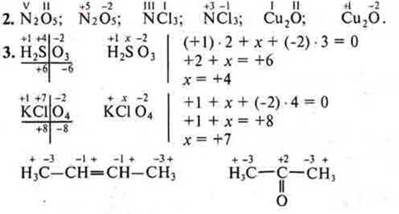
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.