
Changes in the properties of elements and their compounds depending on their position in the Periodic table. The meaning of D. I. Mendeleev's Periodic law and Periodic system of chemical elements
Lesson objectives: to consolidate knowledge of the causes of changes in the properties of elements based on position in the system; to teach how to reasonably explain and compare the properties of the elements, and they formed simple and complex substances; to teach how to give a full description of the chemical element in PSHE; to give an idea of the value of the Periodic law and the Periodic system of chemical elements for the development of science and industry.
Equipment: PSHA of D. I. Mendeleev, totransparency.
Lesson progress
I. Organizational moment
Test of students ' knowledge: chemical dictation on variants; involves quick work using the Mendeleev PSCE.
1. Specify the element whose atom contains:
a) 25 protons a) 41 protons
b) 13 electrons b) 20 electrons
2. Name two elements in whose atom:
three energy levels five energy levels.
3. Determine the two elements that have the last energy level in their atom:
4 valence electrons 7 valence electrons
4. Specify the position of the elements in the PSE.
a) No. 37 a) No. 24
b) # 30 b) # 50
5. what are the similarities and differences in the composition of atoms
6. Give the wording of the Periodic law:
according to D. I. Mendeleev.
II. Learning new material
Plan of presentation
1. Reasons for changing the properties of elements based on the position in the PSE:
a) in periods (small, large);
b) groups of the main subgroup;
2. Changing the properties of chemical elements and their compounds:
a) in periods;
b) groups, major subgroups.
3. the Meaning of the Periodic law and Periodic system of chemical elements of D. I. Mendeleev (possible messages of students).
4. Plan of chemical element characterization based on its position in the PSE.
What are the reasons for changing the properties of chemical elements? What are the reasons for frequency? To answer these questions let's compare the atoms of the elements:
a) No. 11-Na, No. 15-P, No. 17-SL, No. 18-AG;
b) № 3, № 19, № 37.
1. What is the charge of the nucleus of these atoms, what happens to it?
Answer:
a) Na- + 11, P- + 15, Cl - + 17, AG- + 18; the core charge increases gradually towards the end of the period;
b) Li — +3; K - +19; Rb — + 37; the core charge increases rapidly towards the end of the group, the main subgroup.
2. Determine the number of electrons at the external energy level. What is observed?
Answer:
a) Na-1E-; P-5e-; SL-1E-; AG-8E-. The number of electrons at the external energy level increases gradually towards the end of the period.
b) Li-1E-, K-1e-, Rb-1E-. The number of electrons at the external energy level remains unchanged at the end of the group, the main subgroup.
3. How many energy levels in the atoms of these elements that is observed?
Answer:
a) Na-three, P-three, CL-three, AG-three. The number of energy levels does not change, the same.
b) Li-two, K-four, Rb-five. The number of energy levels increases towards the end of the main subgroup group.
4. What do You think happens to atomic radii, as a result of these changes?
a) by the end of the period;
b) to the end of the group, the main subgroup.
Answer:
a) by the end of the period, the atomic radius decreases due to the increased mutual attraction of the atomic nucleus and electrons of the external energy level (work with the table).
b) by the end of the group, the main subgroup, the atomic radius increases because the number of energy levels in the atom increases.
5. do such changes in atomic radii in periods and groups, major subgroups, Affect the ability of atoms to give up electrons, or their attachments?
Answer:
When the atomic radius decreases, the ability of atoms to give up electrons weakens, and the ability to receive electrons increases. By the end of the period, atoms of elements can more easily accept the electrons that ensures the manifestation of nemetallicheskie. When the atomic radius increases, the ability of atoms to give up electrons increases. By the end of the group, the main subgroup, the atoms of elements give up electrons more easily, which ensures the manifestation of metallicity.
In small periods of change in the properties of elements occurs rapidly, in large slow periods, because of atoms being completed privnesli energy level, and in the super-large span (VI, VII) changes occur more slowly, since the lanthanides and actinides being completed not external or predvneshnem, and a third outside level with 18th-to 32е-, whereby the properties of these elements are similar to each other, and these elements form a family of lanthanides, and actinides.
6. what is the reason for periodic changes in the properties of elements? Compare the structure of the atoms of elements # 3, 11, 19, 37. What are their similarities and differences?
Answer:
These are the elements Li, Na, K, and Rb. They are located in the I group of the main subgroup, have the same structure of the external energy level Li-2s', Na-3s', K-4s', Rb-5s'.
However, for each of these elements, the electron of the external energy level is located at a different distance from the nucleus of the atom, so that their chemical activity is different, but their properties are similar. The reason for periodicity is a change in the structure of the external, as well as the pre-external energy level; the repetition of the number of electrons of the external (pre-external) energy level.
Then a short diagram is drawn up as a conclusion for this item:
In periods (by the end of the period)

In groups, major subgroups (to the end of major subgroups)

The frequency of changes in the properties of elements affects the properties of simple substances formed by them and the properties of more complex compounds: oxides and hydroxides.
Students are invited to familiarize themselves with table 6 of the textbook. Especially to work with the change in the properties of simple substances in periods and groups, the main subgroups, to consider in more detail the change in the properties of higher oxides and hydroxyl in periods and groups, the main subgroups.
In periods, the character of the chemical properties of higher oxides changes from basic to acidic, the reason is the same — the atomic radius of the ion decreases.
III
period 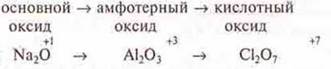
The character of hydroxides changes in the same way.

Thus, by the end of the period in complex connections: oxides and hydroxides-there is a weakening of the basic properties and strengthening of acidic ones.
If we consider the groups, the main subgroups, where there is an increase in metallicity towards the end of the group, we should conclude that there is an increase in the basic character of the oxide, hydroxide, from acidic, through amphoteric.
Example: group III, the main subgroup.
If we consider group I, the main subgroup, then there is an increase in the basic character, since these are typical metals. The hydroxides formed by these elements are the strongest bases-alkalis.
What is the meaning of the Periodic law and the Periodic system?
On this issue, it is possible to report students (prepared in advance), or the teacher focuses the attention of students on such conclusions, which can be recorded on the codotransport.
We can divide the knowledge of the Periodic law into two stages: the first stage — during the life of D. I. Mendeleev, the second stage-modern development.
The first ethane of knowledge
1. Properties of chemical elements, simple substances, the form and properties of compounds of elements are in periodic dependence on atomic weights.
2. the Law is explained on the basis of atomic and molecular theory.
3. A chemical element is understood as a species of atoms with the same relative atomic mass.
4. Made rearrange items:
AG-K, Co-Ni, Te-I. The reason for it has not been established.
5. The reason for the periodicity of element properties is not clear.
Meaning of the law at the first stage:
1. fixed the atomic masses of some elements.
2. a scientific classification of elements based on the periodic law is Given, taking into account their atomic masses and chemical properties.
3. Retold the opening of a number of elements. Described in detail the properties of akabara, aqualumin, ecocrime.
4. Open the inert gases.
The second stage of cognition:
1. Properties of chemical elements of simple substances. The shape and properties of compounds depend periodically on the amount of charge of atomic nuclei.
2. The law is explained on the basis of the theory of atomic structure.
3. a Chemical element is taken as a type of atom with the same charge of the nucleus.
4. the reason for the permutation of AG — K, Co — Ni, Te — I is Explained.
The elements are arranged according to the charge of their atomic nuclei.
The forward element has more heavy isotopes, so its relative atomic mass is greater.
5. the reason for the periodicity of the properties of elements is Explained: the number of electrons at the external (pre-external) energy level is periodically repeated.
Meaning of the law in the second stage:
1. a theory of the structure of the atom has been Created.
2. scientific classification of chemical elements is Given on the basis of the periodic law and the structure of the atoms of elements.
3. the natural boundary of the periodic table is Defined.
4. open elements № 43, 61, 72, 75, 85, 87, 91.
5. Synthesized elements No. 93-109-111.
6. made discoveries in the Sciences: physics, Geology, biology.
7. Significance of the law for educational purposes:
a) significantly reduced time to study the properties of elements, it becomes possible to predict their properties;
b) the belief is formulated that the surrounding world is one, because it consists of the same chemical elements, and it is cognizable.
In this lesson, you should familiarize students with the plan of characteristics of a chemical element according to its position in the PSE. In this characteristic, students demonstrate their knowledge of the periodic law of a Periodic system and their ability to use it correctly.
Plan of chemical element characterization based on its position in the Mendeleev PSC
I. name of the element, chemical sign, relative atomic mass, ordinal number; period number, group number, subgroup — main or secondary.
II. The structure of the atom of an element:
a) the charge of the nucleus of an atom; the number of protons, neutrons in the nucleus of an atom; the number of electrons in an atom;
b) the electronic formula of an atom and an electron-graphic image; a family of s -, p -, d -, f -; a metal or non-metallic element; S. O. — maximum, minimum.
III. External oxide, the nature of the higher oxide; chemical properties of the higher oxide (suggest several reaction equations.
IV. Hydroxide, character of the hydroxide (bases, acid) chemical properties of the hydroxide (make several reaction equations).
V. Hydrogen compound; character of the hydrogen compound (basic, acidic).
VI. give a comparison of this element with the next standing ones by period; by group, main subgroup (its metallicity or nonmetallicity is compared).
As an example for students to consolidate their knowledge, we can offer the characteristics of metallic (CA) and non-metallic (Cl) elements, compiled on the codotransport or printed on cards.
Metal element
I. Calcium (CA); AGCA= 40;
No. 20; IV period; II group, main subgroup.
II. a) +20; 20 protons: 40 + 20 = 20 neutrons;
20 electrons;
b) s-element;
metal element; S. O. +2.
III. Cao-calcium oxide; basic:
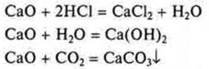
IV. CA(ON)2-hydroxide, base:
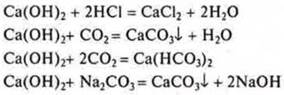
V.![]() -calcium
hydride.
-calcium
hydride.
![]()
VI. Compare the period of CA with K and Sc; the atomic radius of CA is greater than the atomic radius of Sc, but less than the atomic radius of K. Therefore, it is more metallic than Sc, less metallic than K. Compare by group, main subgroup with Mg and Sr; Sa Atomic radius larger than the atomic radius of Mg is smaller than the atomic radius of Sr, CA Metallica magnesium, metallican less than Sr.
Non-metallic element
I. Chlorine (CL); AGCl= 35,5;
No. 17; III period; VII group, main subgroup.
II. a) +17, 17 protons; 18 neutrons; 17 electrons,
b)![]() p-element.
p-element.
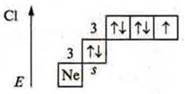
Maximum CP: +7; minimum CP: -1.
SL is a non-metallic element.
III. CL2O7-chlorine oxide (VII), acid oxide.
![]()
IV. HC-4-hydroxide, acid:
![]()
V. H+SL-- hydrogen compound, acidic character.
VI. By period we compare it with S:
Cl is more non-metallic than S, since the atomic radius of Cl is less than the atomic radius of S.
By group, the main subgroup is compared with F and VG.
CL is more non-metallic than VG, since the atomic radius of CL is less than the atomic radius of VG. In comparison with F, the nonmetallicity of Cl is less, since the atomic radius of CL is greater than the atomic radius of F.
III. Homework assignment
Prepare for test work # 1: repeat § 1-5; work out the characteristic of the element on the example of K; N.
IV. Consolidation
PART A. Test tasks with answer selection and correlation.
1. Atomic nuclei were discovered:
A. D. Mendeleev.
By B. E. Rutherford;
B. J. Thomson.
G. D. Chedvig.
2. The period number in the Periodic table is determined:
A. the Charge of the nucleus of an atom.
B. the Number of electrons in the outer layer of the atom.
B. the Number of electron layers in an atom.
D. the Number of electrons in an atom.
3. The shape of the electronic orbitals characterizes:
A. the Main quantum number.
B. the Magnetic quantum number.
B. the Orbital quantum number.
D. Spin quantum number.
4. a Pair of elements that have a similar structure of the external and external energy levels:
A. S and CL.
B. Be and V.
B. Kg and Xe.
G. Mo and Se.
5. the p-element is:
A. Scandium.
B. Barium.
V. Arsenic.
The City Of Helium.
6. Electronic configuration ... 3d104s2corresponds to the element:
A. Calcium.
B. Krypton.
B. Cadmium.
The City Of Zinc.
7. Amphoteric hydroxide is a substance whose formula:
A. Zn(OH)2.
B. Mg(OH)2.
V. Sa(ON)2.
G. SG(ON)2.
8. A number of elements arranged in order of strengthening of steel properties:
A. Mg—Ca—Zn;
B. Al-Mg-CA;
B. Sr-Rb—K;
G. Ge-Si-Sb.
9. Element e with the electronic formula 1s22s22p63s23p63d104s24p1forms a higher oxide corresponding to the formula:
A. E2O.
B. E2O3.
B. EO2.
G. EO3.
10. the isotope of calcium, in the nucleus of which there are 22 neutrons, is designated:
![]()
![]()
11. Establish a match. Element:
I. Aluminum. II. Potassium. III. Selenium. IV. Magnesium.
Electronic formula:
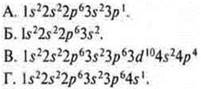
The formula of the highest oxide:
1. E2O. 2. E2O3. 3. ES. 4. EO3.
Higher hydroxide formula:
and. EON. b. E(HE)2. V. E.(ON)3. GN2ЭOIE 4 ..
PART B. Tasks with a free response.
12. based on the position in the Periodic table, place the elements: germanium, arsenic, sulfur, phosphorus — in descending order of oxidizing properties. Explain the answer.
13. How and why do metallic properties change in the Periodic table?
A. Within the period. B. Within the main subgroup.
14. Make an electronic formula for an element with the ordinal number 30 in the Periodic table. Make a conclusion about whether this element belongs to metals or non-metals. Write down the formulas of its higher oxide and hydroxide, and specify their nature.
15. What chemical properties are characteristic of the highest oxide of the element of the 3rd period, the main subgroup of the VI group of the Periodic table? Confirm the answer by writing the reaction equations.
Answers to tasks to consolidate
PART A.
1. B; 2. V; 3. V; 4. V; 5. V; 6. G; 7. A; 8. B; 9. B; 10. G; 11.A - 2, b; B - 3, b; B - 4, g; G - 1, a.
PART B.
12. S-P — As-Ge-decreasing oxidative properties, because the radius of the atoms of elements increases, weakens the connection of electrons of the external energy level with the nucleus of the atom, weakens the ability of atoms to attach electrons — to exhibit oxidative properties.
13. A. within the period (end of period) increases, the nuclear charge, number of electrons in the outer energy level, the atomic radius decreases, reducing the metallicity is the ability of an atom to give electrons. B. within the main subgroup (by the end of the group, the main subgroup), the core charge and the number of energy levels increase. The number of electrons of the external energy level remains unchanged, increasing metallicity — the ability of the atom to give up electrons.
14. No. 30. Zn (zinc). 1s22y22p63s23p64s23d10is a d-element; at the external energy level, two electrons are therefore a metal element exhibiting transitivity, since the d-element.
ZnO-zinc oxide, amphoteric oxide;
ZN(OH)2-zinc hydroxide, amphoteric base.
15. III period, VI group, main subgroup-sulfur, maximum C. O. +6.
The electronic formula is 1s22s22p63s23p4, sulfur is a non-metallic element. S+6O3— higher oxide, sulfur oxide (VI), acid oxide.
a) interacts with water;
SO3+ H2O = H2SO4-sulfuric acid;
b) interacts with soluble bases:
SO3+ KOH = KHSO4-potassium hydrosulfate, acidic salt;
SO3+ 2CON = K2SO4+ H2O-potassium sulfate, medium salt;
C) interacts with the main oxides:
SO3 + CaO = CaSO4-calcium sulfate.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.