
"Types of chemical bonds. Types of crystal lattices" - structure of MATTER - LESSON PLANS for CHEMISTRY 11 class - lesson plans-lesson plans-author's lessons-plan-lesson summary - chemistry
Lesson objectives: to deepen and expand knowledge about the causes of different types of chemical bonding and mechanisms of their formation; to teach us to characterize the chemical bonds according to a certain plan; to give a more complete idea about all types of crystal lattices; teach to correlate the dependence of the physical and chemical properties of substances from a chemical bond and crystal lattice type.
Basic concepts: chemical bond; types of chemical bond; covalent, ionic, bond formation mechanisms: exchange, donor-acceptor; electronegativity, ionic degree ионности, crystal lattices: atomic, ionic, molecular; dipole moment; σ - and π-bonds; bond multiplicity.
Equipment: table "Chemical bond"; totransport "Scale EO on Poling"; models of crystal lattices; an ebony stick; a burette with water, task cards (for fixing).
Lesson progress
I. Organizational moment
The teacher informs students of the results of the test work; analyzes the performance of tasks, analyzes typical errors; you can ask students to do work on errors.
II. Learning new material
Plan of presentation
1. Determination of the chemical bond. The reasons for the formation of the chemical bond.
2. The ionic bond. A type of crystal lattice of compounds with an ionic bond. Physical properties of substances.
3. Covalent bonding. Mechanisms of formation of a covalent bond by MVS: a) exchange; b) donor-acceptor.
4. Varieties of covalent bonding; a) nonpolar; b) polar.
5. σ - and π-connections. Multiplicity of the link.
6. Types of crystal lattices of covalent bond compounds. Physical properties of substances.
7. The concept of "bond polarity and polarity of molecules."
Question. What types of chemical bonds are known? And what are the types of crystal lattices?
Answer. Known covalent bond, ionic bond, metal bond, hydrogen bond. Types of crystal lattices — ionic, atomic, and molecular.
The main objectives of the lesson are to find out the causes of various types of chemical bonds and study the mechanisms of bond formation.
Question. What is a chemical bond?
Answer. Chemical bonding refers to the electrical forces of attraction that hold particles together. Particles can be atoms, ions, or molecules.
The reason for the formation of a chemical bond between particles is the desire of the system to minimize energy. The energy of the resulting system-a chemical bond-is less than that of isolated particles. There is a gain in energy.
Among the particles, the most stable ones are those with the external energy level completed. Noble gases have an octet of electrons at the external energy level, whereas Non — 2e-gases have an octet of electrons . Thus, atoms that have less than 8 electrons at the external energy level tend to acquire the structure of inert gases, i.e., to have an octet of electrons at the external energy level.
The formation of such stability can occur in several ways and leads to the formation of compounds with different types of chemical bonds: covalent, ionic, metallic, and hydrogen.
Any chemical bond is formed only when the convergence of particles (two or more) leads to a decrease in the total energy of the system. The determining factors are the interaction energy — E and the inter-core distance-r.
The most important characteristic factor of an atom when forming a chemical bond is its electronegativity (EO) — the ability to attract electrons.
Next, you need to work with the table " EO Scale byPauling" (totransport). The EA increases by the end of the period, by the end of the main subgroup group.
Defining the connection type:
— if the atoms have the same EO, a covalent bond occurs;
— if the atoms have differentEOS, but do not differ sharply, the difference in EO;
— if the atoms have differentEOS, sharply differ, the difference in EO is > 1,7-an ionic bond occurs.
On the EA scale byPauling can determine the degree of ionization — the polarity of the bond: the greater the difference in the EO, the greater the degree of ionization, the more polar the bond.
|
Difference in the item instance |
Degree of ionization, % |
|
0 |
0 |
|
0,5 |
6 |
|
1,0 |
22 |
|
1,5 |
44 |
|
2,0 |
63 |
|
2,5 |
79 |
|
3,0 |
89 |
Thus, if the difference in EO is greater than 1.7, an ionic bond occurs.
Ionic bonding-bonding due to the electrostatic attraction of oppositely charged particles (cations — positively charged and anions-negatively charged). Ionic bonding occurs between atoms that differ sharply in EO — typical metals and typical nonmetals. Consider the mechanism of formation of an ionic bond in a NaCl compound.
This compound was formed between the Na atom — a typical metal, EONa = 0.9, and the CL atom is a typical nonmetal, EOCl= 3.2.
The difference in EO = 2.3, therefore, there is an ionic bond.
When Na interacts with SL2:
2Na + Cl2 = 2NaCl
as a result окислительноof the redox reaction, cations of a strongly electropositive element (metal) and anions of a strongly electronegative element are formed.
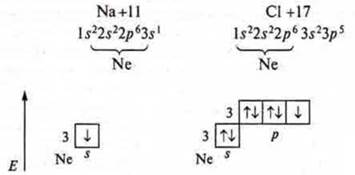
The recoil of one s-electron of the Na atom is observed:
![]()
There is a stable particle with an octet of electrons at the external energy level: 1s22s22p6-Na+, sodium cation. The chlorine atom attaches an electron to the p-sublevel:
![]()
There is a stable particle, just as with an octet of electrons at the external energy level-the chlorine annon. Between the formed ions, there are forces of electrostatic attraction that will keep them near each other, thereby making an ionic bond. It is characteristic of binary compounds formed by metals and non — metals, as well as more complex — three-element-compounds: alkalis, salts. In this case, the cations and anions can be not only simple, but also complex.
Example: ammonium cation-NH4+; sulfate-anion SO42-; hydroxide-anionOH -.
Cations and anions interact with each other to form substances in a solid state with an ionic crystal lattice. In the space of the cations and anions are arranged in an orderly manner. The smaller the size of the ion and the larger its charge, the stronger its electrostatic field and the stronger the chemical bond.
Thus, the energy of the LiF crystal lattice is 1004 kJ/mol, and that of NaCl is 755 kJ/mol. All substances with an ionic crystal lattice are volatile, solid, and refractory, and conduct electric current in solutions and melts. However, crystals with an ionic bond — ions-are very brittle, because with a slight shift in the planes in the crystal, there is a close approximation of similarly charged ions, which, pushing off from each other, cause a break, a crack appears in the crystal.
![]()
At high temperatures, many substances with an ionic bond, such as halides, pass into gaseous compounds. The gas phase can contain NaCl molecules and their aggregates (NaCl)2 with unstable covalent bonds, as well as Na+ and Сl-ions.
It should be known that compounds with an ionic bond are a limited number, and there is no pure ionic bond in compounds. In these cases, we should talk about the degree of ionization. The higher it is, the more ionic the bondis .
As the number of electrons increases at the external energy level of the metal atom, the strength of their bond with the nucleus of the atom increases, and the ability to form an ionic bond decreases. Aluminum has covalent bonds with halides, but under the influence of, for example, polar solvents, these bonds become ionic.
Ionic bonding is an extreme case of covalent polar bonding. What is a covalent bond?
Communication by means of common electron pairs is called covalent. It occurs if the difference in the EA is less than 1.7 and approaches zero. If the atoms have the same EO, a covalent nonpolar bond occurs; if the atoms have different EO, a covalent polar bond occurs.
There are two mechanisms of covalent bonding: exchange and donor-acceptor. The following conditions are required for an exchange mechanism:
1) interacting atoms must have unpaired electrons characterized by different spin quantum numbers:
![]()
2) the system should contain a particle with a low energy reserve, which would absorb the energy released during the formation of a chemical bond, since the formation of a bond is an endothermic process:
![]()
Particle X can be either A or B with a low energy reserve. Having received energy, it can participate in the formation of a chemical bond itself.
This mechanism of formation of a chemical bond is explained by the method of valence bonds (MVS).
Example: formation of a hydrogen molecule H2:
hydrogen
atom — ![]()
hydrogen
atom — ![]()
radius of the atom H-rh = 0.053 nm
When a chemical bond is formed, the AO (atomic orbitals) overlap.
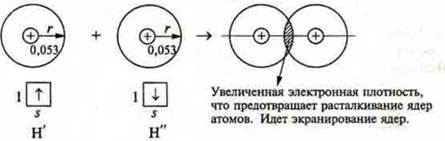
If there were no overlapping orbitals, the inter-core distance would be 2r = 0.053 x 2 = 0.106 (nm).
However, in a hydrogen molecule, this distance is equal to 0.074 nm, i.e. less. Therefore, there is an overlap of orbitals.
The approach of hydrogen atoms N' and N" the forces of electrostatic attraction of the electrons of atom N' to the nucleus of the atom, N" and electrons the atom N" by the nucleus of the atom' N ' will increase, will begin to attract to each other. At the same time, the repulsive forces between the similarly charged nuclei of the H' and H ' atoms will increase. This leads to the fact that atoms can approach each other so that attraction forces are completely balanced by the forces of repulsion and electron clouds begin to overlap, allowing electrons of a single atom N' in the attractive field of the nucleus of an atom of N" and Vice versa, and every atom in some point will have a completed outer energy level, like noble gases.
A bond formed by the formation of common electron pairs that equally belong to both atoms is called covalent.
Types of covalent bonding:
a) a nonpolar covalent bond occurs between atoms that have the same EO.
Example: O2; O3; N2; CL2; H2, etc.
b) a polar covalent bond occurs between atoms that do not differ sharply in EO.
Example: NCl, NH3, H2O, etc. (if the time of the lesson allows in classes where students perceive the material more consciously, you can explain the concept of "dipole moment" to explain the polarity of the connection).
The dipole moment μ is the product of the length of the dipole / (the distance between two charges equal in magnitude and opposite in sign + q and -q) by the absolute value of the charge: μ = lq.
The dipole moment is a vector value and is directed along the dipole axis from a negative charge to a positiveone .
Example:
HF, μ = 6,4 · 10-30
NSl, μ = 3.5 · 10-30
HBr, μ = 2.6 · 10-30
HI, μ = 1,3 · 10-30
The greatest dipole moment of hydrogen fluoride, where F has a greater EO.
The molecule H2O μ = 6.1 · 10-30.
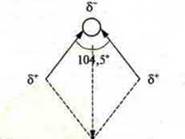
Angular structure of a molecule
The CO2 molecule,
![]()
Each bond is polar and MK = 9 * 10-30, but the molecule is nonpolar, MK = 0, because the bonds C=0 are located on the same line and compensate for each other's dipole moments. Why the CO2and H 2 O molecules have such a structure in space2is a question of the following lessons of the topic.
From the above, it is necessary to distinguish the concepts of "bond polarity" and "molecule polarity" in General (demonstration experience). In simple diatomic molecules, the dipole moment and polarity of the bond are equal, but in complex compounds H2O , NH3, HCl, CO2— are not equal.
In cases of difficulty in understanding the dipole moment, it is possible to explain the polarity of the bond by finding the difference in the EO of the atoms forming the bond, and then to conclude which bond is more polar.
Example: HF and HI
![]()
ПолярнееThe connection in the HF connection is more polar, since the difference in the EO of 1.9 is greater than 0.4.
A polar covalent bond is an intermediate one between a nonpolar covalent bond and an ionic bond.
In addition to the exchange mechanism of covalent bond formation, a donor-acceptor mechanism is also possible.
For the implementation of such a mechanism, the following conditions are necessary:
1.
there should be two particles In the system. One is a donor who has an
undelivered pair of electrons.![]() dRuga
is an acceptor with a free orbital
dRuga
is an acceptor with a free orbital ![]()
Such particles are formed as a result of an exchange mechanism, when electrons are redistributed.
Example:
![]() —
free orbital, acceptor (H+SL)
—
free orbital, acceptor (H+SL)
![]() —
undelivered pair of electrons, donor (K+N-)
—
undelivered pair of electrons, donor (K+N-)
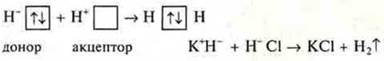
2. The system needs to be particle absorbing energy (as with the exchange mechanism).
Example: formation of an ammonium ion.
[NH4]+; ammonia, NH3, N +7.
1s22s22p3
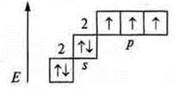
![]() -
three connections are formed by the exchange mechanism: a nitrogen atom has an
unassigned pair of 2s2 electrons, a nitrogen atom is a donor, a
hydrogen cation
-
three connections are formed by the exchange mechanism: a nitrogen atom has an
unassigned pair of 2s2 electrons, a nitrogen atom is a donor, a
hydrogen cation ![]() -
acceptor
-
acceptor
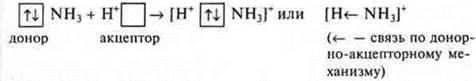
The multiplicity of the connection corresponds to the number of common electron pairs. Single bond — one shared electron pair, a double bond — two shared pairs, a triple — three shared pairs.
When the orbitals overlap axially, a strong, non - polarizable, hard-to-break трудноразрываемая σbond (Sigma bond) occurs.
When the s -orbitals overlap axially , σis the s—s bond:
![]()
When the p -orbitals overlap axially , σis the p—p bond:
![]()
When the s -orbitals and p -orbitals overlap axially, σis the s—p bond:
![]()
In the case of a double or triple bond, in addition to forming a σ -bond (axial overlap of orbitals), a π-bond (PI-bond) is also formed. In this case, the orbitals overlap laterally орбиталей.
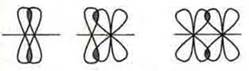
π-link
In the case of a double bond — one σ-bond, another π-bond; triple bond — one σ-bond and 2 π-bonds.
In some cases, it is possible to find a bond in a dynamic state, i.e. electronic clouds are "smeared" between two, three, four, etc. atoms, then the multiplicity of the bond is fractional, one and a half. In benzene With6H66E- - π-cloud, the bond is one and a half, if the single bond has a length of 0.154 nm, the double bond-0.134 nm, then in the aromatic ring its length is 0.140 nm.
All substances with a covalent bond can usually be liquids, gases, solid in the aggregate state; low-melting, volatile. They can form two types of crystal lattices.
Atomic crystal lattices — in the nodes of the crystal lattice there are atoms with covalent bonds between them.
Example: diamond, graphite, boron, silicon,
SiC-carborundum; SiO2-quartz; some silicides, carbides, oxides: Al2O3; SG2O3; physical properties of substances with an atomic crystal lattice — solid, refractory, non-volatile, insoluble in water.
Molecular crystal lattices — the nodes contain molecules with weak forces of intermolecular interaction between them. Most substances with such a lattice are gaseso 2; N2; CO2; CL2, liquids-water, alcohol, acids, VG2; solids — naphthalene, I2, petroleum, glucose, sucrose. They are volatile, brittle in their crystalline form, and have a lowt boiling point and melting point. Depending on the polarity of the molecules, they can be soluble in water , dissociate, and conduct an electric current.
III. Generalizations and conclusions on key issues of the topic
Thus, we found out the causes and mechanisms of formation of ionic and covalent bonds of no MVS, and also noted the dependence of the physical properties of substances on the type of chemical bond, the type of crystal lattice.
Students can be shown a demonstration experience that confirms the ability of polarized molecules to Orient themselves in an electric field and move to a high voltage region.
Fur rubs an ebony stick, which approaches a thin stream of water flowing out of the burette. The jet is attracted to the stick. A stream of benzene or ether will not be attracted to an electrified stick.
IV. Consolidation
1. Determine the type of chemical bond in compounds. Justify the answer:
Vasl2; SO2; C2H6; F2; KVr; I2.
2. in which of these compounds is the most polar connection? Arrange connections in ascending order of polarity:
HCl; F2; Н2O; NH3; H2S
3. what is the reason for the sharp difference in the physical properties of CO2and SiO2?
4. How is the σ -bond and the π-bond formed?
The answers to the questions of consolidation
1. Ion bonding: KVr; Vasl2. The elements that make up a compound differ sharply in the EO. Covalent polar coupling.
SO2; C2H6-elements forming compounds do not differ sharply in the EO. Non-polar covalent bond.
F2; I2— the elements forming the compounds are identical in EO.
2. Most polar bond in the compound H2O.
EOn= 2.1; E0O= 3.5, the difference in EO is 3.5 - 2.1 = 1.4.
![]()
3. CO2-covalent polar coupling: gas. Solid — molecular crystal lattice, low-melting, volatile;
SiO2-covalent polar bond, solid, refractory; atomic crystal lattice.
4. σ-bond occurs when the orbitals overlap axially, strong, difficult to polarize.
the π-bond occurs when the orbitals overlap laterally. it is mobile, easily polarizable, and unstable.
IV. Homework assignment
1) § 6 p. 44-51. № 3
2) draw up schemes for the formation of a chemical bond in the compounds KN, NH3, HF; specify the type of chemical bond, mechanism, formation, type of crystal lattice, physical properties of substances, (do your homework on a separate sheet).
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.