
Theory of chemical structure of organic compounds of A. M. Butlerov. Modern directions of theory development - structure of MATTER - LESSON PLANS in CHEMISTRY 11 class - lesson plans-lesson plans-author's lessons-plan-lesson summary - chemistry
Lesson objectives: to compare the basis of commonalities of the two leading theories of chemistry: the theory of periodicity Mendeleev and the theory of the structure of A. M. Butlerov: opening presents theories of the structure of the compounds proposed by the predecessors of A. M. Butlerov; the theory of radicals, the theory of types; all the background theory of the structure of chemical compounds; to consolidate knowledge of the main provisions of theory of chemical structure; learn to use them in describing the structure of compounds not only in organic but also inorganic.
Basic concepts: radical theory, type theory, chains of carbon atoms — open, branched, closed, bonds — single, double, triple; isomerism: structural, spatial, interclass.
Equipment: tables of the structure of organic compounds, ball-bearing models of metal, ethane, ethine, crystal lattices (models), codotransporants (tables of types, tables of radicals, tasks), phenol , R—R Br2, R—R I2, NaOH.
Lesson progress
I. Organizational moment
The teacher analyzes the results of independent work, focuses the attention of students on typical mistakes. Homework: the type of hybridization and particle geometry are checked verbally.
II. Learning new material
Plan of presentation of the material
1. Comparison of two leading theories of chemistry: D. I. Mendeleev's Periodic law and A. M. Butlerov's theory of chemical structure.
2. the Main commonalities of the two leading theories of chemistry.
3. The first theory of the structure.
4. The second premise of the theory structure.
Isomerism, types of isomerism.
5. the Third position of the theory of structure is the mutual influence of atoms in the molecules of substances.
Consider the example of phenol.
III. Introductory conversation
The periodic theory of D. I. Mendeleev and the theory of chemical structure of A. M. Butlerov were created by great Russian scientists and constitute the contribution of Russian chemistry to the world chemical science. They have passed the test of time and passed it brilliantly, developing and enriching themselves with modern chemical discoveries. On totransparency the recorded sayings of the scholars, they are considered prophetic.
"The future does not threaten the periodic law with destruction, but only development and superstructures are promised" (D. I. Mendeleev).
"It goes without saying that when we know more intimately the nature of chemical energy, the very nature of atomic motion — when the laws of mechanics are applied here also-then the doctrine of chemical structure will fall, as the former chemical theories fell. But like most of these theories, it will fall not in order to disappear, but in order to enter in a modified form into the circle of new and broader views" (a.m. Butlerov).
The teacher invites students to compare the basics of commonality of the two leading theories of chemistry.
Comparison of two leading theories of chemistry
|
Flag |
D. I. Mendeleev's periodic law |
Theory of the structure. A. M. Butlerov |
|
Opening time |
1869 |
1861 |
|
Background |
63 elements were known and the properties of their numerous compounds were described |
Many organic compounds are known — hundreds of thousands consisting of atoms; C, H, O, rarely N, P, and S |
|
Works of predecessors |
Classification of J. Berzelius (metals and non-metals): I. V.Decramer (triad); D. A. R. Newlands (octaves); L. Meyer |
J. J. Dumas (radical theory); J. Dumas, S. Gerard, O. Laurent (type theory). J. Berzelius introduced the term "isomerism"; F.Veler, N. N. Zimin, M.Berta, A. M. Butlerov (synthesis of organic substances, the collapse of vitalism): F. A. Kekule (structure of benzene) |
|
D. I. Mendeleev's periodic law |
Theory of the structure. A. M. Butlerov |
|
|
The Congress of chemists in Karlsruhe in 1860 |
D. I. Mendeleev was present in the role of observer |
A. M. Butlerov was not involved (but actively studied the materials of the Congress). He took part in the Congress of physicians and naturalists in Speyer in 1861, where he made a report "on the structure of organic bodies» |
|
Personal qualities |
Both authors differed from other chemists: an encyclopedic chemical knowledge, ability to analyze and summarize factual, scientific forecasting, Russian mentality and Russian patriotism. D. I. Mendeleev about A. M. Butlerov: "A. M. Butlerov is oneof the greatest Russian scientists, he is Russian both for his academic education and for the originality of his works» |
|
|
The role of practice in the formation of the theory |
D. I. Mendeleev predicts, describes and indicates the ways of discovery of gallium, scandium and germanium that are still unknown to science |
A. M. Butlerov predicts and explains the isomerism of organic compounds, himself performs many syntheses |
|
The main directions of development of the theory (along the spiral) |
"The properties of chemical elements and substances formed by them are in periodic dependence on their relative atomic masses» «...From the charges of their atomic nuclei» "From the periodicity in changing the outer electron layers of atoms» |
"The properties of substances depend on the order of joining atoms in a molecule - on their chemical structure»
"From their spatial structure» "From their electronic structure» |
Note. Based on the book: O. S. Gabrielyan. Chemistry: 11th grade: the teacher's Table book. CH. I., bustard. 2003. Pp. 110-111.
Students, working with the textbook and the text of the notes of chemistry lessons in the 10th grade, formulate and disclose the first position of the theory of chemical structure.
Atoms in molecules of organic and inorganic substances are connected in a strictly defined sequence, according to their valence. Carbon in all organic compounds and most inorganic compounds always has valence IV; oxygen-II, nitrogen-III, hydrogen-I.
ЧетырехвалентностьStudents explain carbon tetravalence by considering the structure of the carbon atom in the main and in the excited state based on knowledge about hybridization of atomic orbitals.
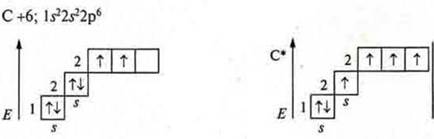
The carbon atom has four unpaired electrons in the excited stateорбитали, with s - — - one and p — - - three in the orbitals.
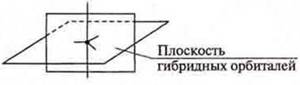
When hybridizing sp3, four hybrid orbitals are formed, the angle is 109°28'. When sp2-hybridization is formed , three hybrid orbitalsare formed, the angle is 120° , and there remains one non-hybrid p-orbitallocated in space in a plane perpendicular to the plane of the hybrid orbitals.
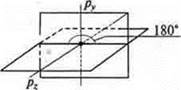
During sp-hybridization, two hybrid orbitals are formed, an angle of 180°, and two non - hybrid p-orbitalsare left, located in space in mutually perpendicular planes.
Carbon atoms in organic compounds form chains of carbon atoms, the valence is indicated by a dash:
a) open:
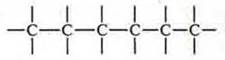
b) branched:
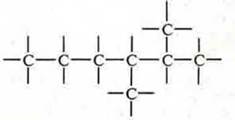
C) closed systems:
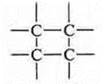
Block diagram:
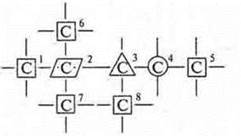
It is necessary to remember the concepts: primary, secondary, tertiary, Quaternary carbon atom. It all depends on how many carbon atoms a given atom is connected to. This compound has 8 carbon atoms.
Carbon
emissions № 1, 5, 6, 7, 8 — primary data, ![]()
No.
4-secondary, ![]()
#
3-tertiary, ![]()
No.
2-Quaternary, ![]()
You can conditionally enter the designations of such atoms.
Depending on the hybridization ofAI AO between carbon atoms can be formed:
a) single bond at sp3-hybridization, bond length 0.154 nm σ-bond:
![]()
b) double bond in sp2-hybridization, one σ-bond, the other π-bond, the length of the double bond is 0.134 nm:
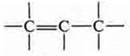
C) triple bond in sp-hybridization, one σ-bond and two π-bonds, the length of the triple bond is 0.120 nm. the shortest connection:
![]()
Based on the first position of the compound, we can make a structural formula taking into account the valence of the atoms forming it, and if carbon atoms, then taking into account its hybridization.
Task: Create structural formulas for compounds
organic:
alkane
C4H10: 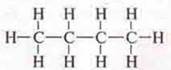
semi-structural formula: H3S-SN2—SN2—SN3
alkene
C4H8: 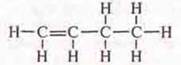
semi-structural formula H2S=SN-SN2—SN3
alkina
S4N6: 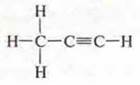
semi-structural formula H3S-S≡SN
and inorganic ones:
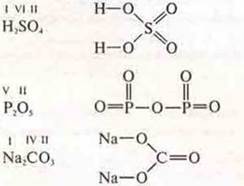
Carbon atoms, having different hybridization, form three simple allotropic modifications with atomic crystal lattices:
Almaz-sp3-hybridization;
graphite - sp2-hybridization: policemen, fullerenes;
carbyne — the sp-hybridization.
Task: The composition of organic matter With2H6O. Suggest structural formulas that are semi-structural to this compound.
First
formula: H3S-O-CH3 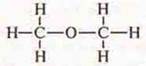
All hydrogen atoms. An equivalent oxygen atom is the EO the same effect on the atoms of carbon and hydrogen. This is ether: a gas, low boiling point-23.6°, soluble in water (in 1 volume of water at 18° 37 volumes of ether are dissolved), does not interact with metallic sodium.
Second
formula: 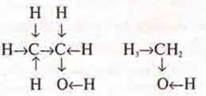
In this compound, there is an unequal hydrogen atom, it is directly connected to the EO by an oxygen atom, which has a stronger effect on it than on the remaining hydrogen atoms connected to the carbon atom.
Bonds-arrows show the displacement of the electron density in the compound to the EO oxygen atom. It is an alcohol that is highly soluble in НH2OO and reacts with Na.
Conclusion: Ether and alcohol are of the same qualitative and quantitative composition, but they have different properties. How can I explain this?
It turns out that the properties of substances depend not only on their qualitative and quantitative composition, but also on their chemical structure. We have seen this in a concrete example. These compounds were called isomers. From the Greek isos-equal and meros-share, part. This phenomenon was discovered as early as 1823 by K. Liebig and F. Freud.Wehler on the example of salts of two inorganic acids: cyanic N-O-C≡N and rattlesnake N-O-N≡C. In 1830, J. Dumas spread the idea of isometry on an organic compound. The term "isomer" was suggested by J. Berzelius. However, this discovery was not given special significance, because in organic chemistry there was complete chaos. am Butlerov gave a scientific explanation of the phenomenon of isomerism in the framework of the theory of structure, while neither the theory of types nor the theory of radicals revealed the essence of this phenomenon. Butlerov saw the reason for the isomerism inthis . because theatoms in the isomer molecules are bound in different order. Based on the structure theory of a.m. Butlerov, it is possible to predict the number of possible isomers and their structure. Experimentally it was proven by A. M. Butlerov and his followers.
Let us formulate again the second position of the structure theory.
The properties of substances depend not only on which atoms of which elements and how many of them are included in the structure of the molecule, but also on how they are connected to each other.
In a further conversation with students, the types of isomerism are discussed: structural isomerism and spatial isomerism.
The types of structural isomerism are: carbon skeleton isomerism; isomerism of the position of the multiple bond of the functional group, substituents; interclass isomerism.
The types of spatial isomerism are geometric qiC- and TRANS-optical isomerism.
III. Performing tasks (on totransparency)
Task: Determine the types of isomerism, comparing compounds, give names.
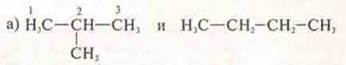
Answer: 2-methylpropane; n-butane;
structural, carbon skeleton;
![]()
Answer: butene-2; butene-1;
structuralthe position of multiple bonds;
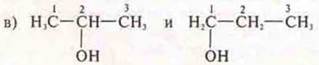
Answer: propanol-2; propanol-1;
structural, position of functional gr.;
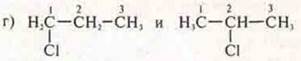
Answer: 1-chloropropane; 2-chloropropane;
structural isomerism, position of the substituent;
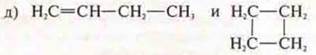
Answer: butene-1; CYCLOBUTANE;
structural isomerism, inter-class;
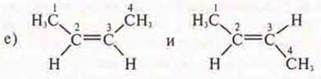
Answer: CIS-butene-2; TRANS-butene-2;
spatial, geometric;
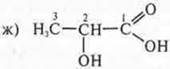
Answer: lactic acid, 2-hydroxypropane kilota.
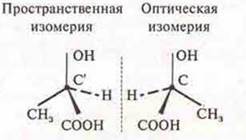
Optical isomers of lactic acid
Consider, recall the third position of the theory of structure about the mutual influence of atoms in the molecules of substances.
The properties of a substance depend not only on the structure and order of joining of atoms in a molecule, but also on their mutual influence on each other.
The presence of functional groups in compounds, substituents affects the characteristics of the properties of substances. Consider the mutual influence of atoms and groups of atoms in a phenol molecule WITH6H5-OH. To explain this phenomenon, let us recall such experiments.
Experience 1. Interaction of phenol with VG2. In a test tube to the phenol pour bromine water (if there is no bromine water, the experiment with solution I2 goes well). There is a yellowish precipitate with bromine water, and with solution I2, the yellow color disappears. Phenol interacts with both VG2and I2.
Experience 2. To a pinkish emulsion of phenol surging sodium hydroxide, shaken. The solution becomes transparent. Phenol interacts with sodium hydroxide, showing acidic properties. Why? Consider the mutual influence of atoms in a phenol molecule.
a) influence of the group-ON on the aromatic ring.
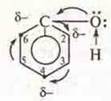
Unshared pair of electrons of the oxygen groups —OhH interacts with the p-electron cloud of the aromatic ring, resulting in a redistribution of electronic density in the ring, the highest electronic density is obtained by carbon atoms under Nos. 2, 4, 6. This makes it possible to easily replace hydrogen atoms with halogen atoms (experiment 1);
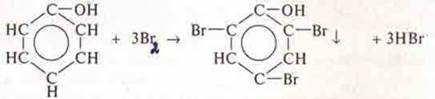
Substitution reaction
b) influence of the aromatic ring on the group-ON.
Due to the fact that the electron pair of the oxygen atom comes into contact with the π -electron cloud of the aromatic ring, the oxygen atom loses its electronic density, in order to make up for it, the General electron pair with the hydrogen atom shifts in its direction. This ensures its mobility, the phenol becomes able to give off a proton, show acidic properties (experiment 2). Acid is much weaker than carbonic acid.
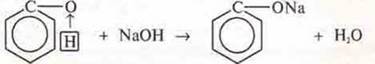
Carbonic acid can displace phenol and sodium phenolate.
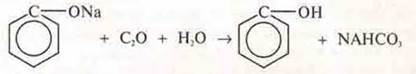
IV. Summary and conclusions
Thus, in this lesson, we have generalized, consolidated at a higher scientific and theoretical level, knowledge of the theory of the structure of compounds by a.m. Butlerov. What is its universality, we will consider in the next lesson.
The main conclusion to be drawn is as follows.
According to the structure of the substance, the properties of this substance can be assumed:
a) presence of only σ-bonds — substitution reaction;
b) presence of π-bonds-joining reaction;
C) the presence of an element with a maximum C. O.-oxidative properties;
d) the presence of an element with minimal CP — reducing properties;
e) presence of an element with intermediate S O and oxidative and reducing properties;
e) presence of an element with a high electronic density, having unsheltered electronic pairs:
:Eδ- - gives the main properties, as well as acidic properties-splinterability to chip off H+(presence of a strong polarity)
Withδ-N
For example, in a row with2H6; with2H4; with2H2, acidity increases, because the hybridization of the carbon atom changes, the bond length decreases, the bond of hydrogen with the carbon atom weakens, and the ability to be replaced increases.
IV. Homework assignment
§ 9 (up to p. 80); Nos. 5, 6; compare the basic properties of CH3—NH2and C6H5—NH2.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.