
Plastics. Elastomers. Fibers. Biopolymers - the structure of MATTER - LESSON PLANS for CHEMISTRY 11 class - lesson plans-lesson plans-author's lessons-plan-lesson summary - chemistry
Lesson objectives: five the idea of classification of polymers on the properties; know the concepts of "plastic", "elastomers", "fiber", to introduce some plastics, fibers, elastomers and learn to identify Navy; consolidate knowledge of biopolymers: characteristics of their structure, qualitative detection, applications.
Basic concepts: plastics, elastomers, fibers, natural polymers-biopolymers.
Equipment: collections of "Plastics", "Fibers", tables: "protein Structure", "Nucleic acids"; alcohol lamp, matches, burning spoon; test tubes, universal indicator.
Lesson progress
I. Organizational moment
The lesson is a continuation of the lesson on "Polymers", where students get acquainted with the second classification of polymers — make sure of the qualitative definition of polymers experimentally in order to prepare for the upcoming practical work. The work can be performed in the form of laboratory or group work.
II. Front-facing conversation
At the beginning of class to conduct a frontal conversation on the issues recorded on totransparency or printed.
1. To define the Navy.
2. What is a monomer? A structural link? Degree of polymerization?
3. Why is the structural link of polyethylene considered to be —CH2-CH2—, and not —CH2-? What is the common and what is the difference between a monomer molecule and the structural unit of the polymer formed by it?
4. Find the structural link of the polymer and determine the structural formula of the monomer.
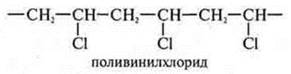
5. Menvironments. polyethylene= 500. Calculate the degree of polymerization. Why does a polymer record an MP?
6. How to explain the lack of volatility is the Navy?
7. Explain the crystallinity and amorphousness of the polymer.
8. What characteristics should be characterized by substances entering the reaction:
a) polymerization? b) polycondensation?
9. List the special properties that characterize most polymers.
Answers to questions from the front-end conversation
1. IUD-substances with a very high molecular weight, containing a repeating group of atoms.
2. Monomer — a low-molecular-weight substance from which IUD is synthesized.
The structural link is a repetitive group of atoms in the macromolecule of the IUD.
The degree of polymerization is a number that indicates the number of structural units in the IUD macromolecule.
3. the monomer of polyethylene is ethylene H2S=CH2, and the structural link is similar in composition to the monomer, but differ in structure, so the structural link of polyethylene is CH2—CH2-.
4. A structural unit of the polymer:
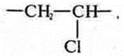
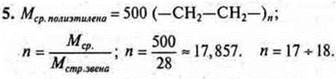
During polymerization, a different number of monomer molecules are combined into macromolecules, depending on when the growing polymer chain breaks. As a result, macromolecules of different lengths and therefore different masses are formed. Therefore, the molecular weight usually indicated for such a substance is only its average value, from which the mass of individual molecules deviates significantly in one direction or another.
6. The lack of volatility of IUD is explained by the fact that macromolecules have a high molecular weight, between them there are significantly greater forces of mutual attraction than those of low-molecular substances. Macromolecules are attracted to each other by a huge number of links.
7. Crystallinity — ordered (parallel) arrangement of macromolecules.
Amorphous — lack of order of macromolecules.
The degree of crystallinity indicates the percentage of ordering of the arrangement of macromolecules. Characterizes the strength of the polymer.
8. Substances entering the polymerization reaction must have multiple bonds.
Substances entering the polycondensation reaction must have functional groups interacting with each other.
9. Most polymers are characterized by: high crystallinity, high mechanical strength, low density-light, chemical resistance-withstand the action of oxidants, acids, alkalis, are not exposed to the external environment; do not have a certain melting point; poor solubility — most swell in solvents.
II. Learning new material
Plan of presentation
1. Classification of polymers by properties and application:
a) plastics; b) elastomers; C) fibers.
2. Biopolymers:
a) proteins;
b) polisaharida:
C) polynucleotides.
3. Laboratory experience. Experimental determination of polymers:
a) polyethylene.
b) PVC.
C) polystyrene.
d) organic glass;
d) nylon;
e) Dacron;
g) wool;
h) cotton;
I) acetate.
K) starch, protein.
Plastics are structural materials containing a polymer that is in a visco-fluid state when forming a product, and when it is used — glassy. In addition to the polymer, the plastic consists of components: plasticizers, stabilizers, dyes, fillers. When heated, the plastics acquire a given shape.
Fillers — wood flour, fabric, asbestos, fiberglass, etc. Improve mechanical strength, reduce the cost of the polymer.
Plasticizers are high-boiling esters. They increase elasticity and eliminate brittleness.
Stabilizers-antioxidants, light stabilizers. Contribute to reducing the properties of polymers in the process of their processing and use.
Dyes give the material the desired color and commercial appearance.
You should know whether the polymer is thermoplastic or thermosetting. Based on polymers: polyethylene, polystyrene, polyvinyl chloride, polypropylene, polymethylmethacrylate-based production of plastics.
Students get acquainted with plastics based on these polymers, working with the collection of "Plastics", describing their appearance (foam, artificial leather, linoleum, plastic, textolite, organic glass).
Elastomers (rubbers) are polymeric materials capable of highly elastic and reversible deformations in a wide temperature range.
Classification of elastomers:
a) natural; b) synthetic.
Rubber (from the Indian words "KAU" - tree, "Uch" — to cry, to flow) - so the Maya called the hevey tree, which when repeatedly incised with the bark "cried" with milk juice-latex (Latin. «liquid»). The name of the tree was transferred by the discoverers to a mass coagulated from latex - rubber. Many plants emit latex: dandelion, ficus.
The important properties of rubber-elasticity and adhesive ability-are the basis for its application for the production of rubber. Other uses of rubber are also known — the production of waterproof fabric, because rubber does not pass water and air. In 1823, K. McIntosh organized the production of waterproof fabric for raincoats impregnated with rubber in Glasgow. They became known as Macintoshes. And 50 years ago, we found out that what is written in pencil is easier to erase with natural rubber. They began to make washing elastic bands. However, at low temperatures, the rubber becomes brittle, and when the temperature rises, it softens. These disadvantages affected clothing made of rubber-impregnated fabric, it lost its wear resistance. In 1839, the American C. Goodyear developed a method for vulcanizing rubber, as a result of which the twisted molecules are "stitched together into a structure of spatial structure" — "volumetric". Rubber is formed. Unlike rubber, it swells in solvents, but does not change its properties when the temperature changes.
Natural rubber formula:
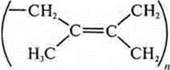
In 1910, S. V. Lebedev obtained the first rubber-like polymer from divinyl.
![]()
The raw material for divinyl was ethyl alcohol.
In the early 30s, industrial production of synthetic rubber was established. Currently, they produce isoprene, butadiene, styrene-butadiene, chloroprene, polyurethane, silicone, acrylic, ethylene propylene rubbers, etc.
Many of them are applicable for manufacturing implants:
- bones and joints-polyacrylates; polyurethanes;
— the joints of the fingers — polysiloxane, polyethylene;
- heart and its parts-polyurethane; polysiloxanes, etc.
— blood vessels-polypropylene, polytetrafluoroethylene. Students get acquainted with elastomers from the collection "Plastics", "Elastomers"; describe the appearance.
Fibers are flexible and strong IUDs of limited length and small transverse dimensions, suitable for the manufacture of yarn and textiles.
Classification of fibers:
a) natural:
— vegetable origin-cotton, flax, hemp;
- animal origin-wool, silk; and non-animal origin-asbestos;
b) chemical:
- artificial — acetate, viscose;
— synthetic — nylon, polyester, acrylic, polypropylene.
Students consider fabrics based on fibers: cotton (cotton); wool, acetate, viscose, Dacron. Describe their appearance.
Biopolymers are IUDs of living nature.
Classification:
a) polysaccharides: starch, cellulose. A sign of polysaccharides-undergo hydrolysis:
![]()
The structure of macromolecules provides properties: linear (cellulose) does not dissolve in H2O2O due to the strong intermolecular interaction; branched (starch) do not dissolve in water, are prone to the formation of gels.
Polysaccharides have a huge role in the cell: energy reserve, support form: biologically active substances, huge nutritional value;
b) proteins of the Navy, are synthesized in the cells. Monomers are amino acids. It is known that the polypeptide chain of a protein (primary structure) is twisted into a spiral (secondary structure), this spiral is rolled into a ball (tertiary structure). And if several tertiary structures are combined into one large structure, it is a Quaternary structure that has biological activity.
Protein molecules are fibrillar — fiber-like and globular-in the form of a ball.
The molecules of fibrillar proteins are elongated, grouped together next to each other, forming super-spirals that resemble ropes or cables due to hydrogen bonds. They are not soluble in water, they are the structural material of cells (carotene of hair, nails, feathers, tendon and muscle collagen). Globular protein molecules are connected in compact glomerules, are soluble in water, in dilute acids, alkalis, and salts, but are deposited in concentrated solutions. They are involved in regulating the cell's vital processes (hemoglobin, enzyme, and hormones).
Protein properties: hydrolysis to simple proteins and amino acids, denaturation — destruction of their structures.
Color reactions: burlova — red-purple staining in the alkaline solution of the surging sea(HE)2in an alkaline environment. Reaction to peptidnuu connection.
Students conduct an experiment with the soluble protein of a chicken egg with si(ON)2; conduct the burning of wool yarn-solid protein, determine the smell of burnt feathers, as well as the deposition of protein (denaturation) by concentrated acids of heavy metal salts (CuSO4).
C) nucleic acids — biopolymers, the monomers of which are the remains of nucleotides. Two nucleic acids are known. DNA is a deoxyribonucleic acid, a carrier of information about the structure of a protein, in the nucleus of a cell in chromosomes. RNA is a ribonucleic acid both in the nucleus and in the cytoplasm, responsible for the transfer of information about the structure of a protein with DNA and RNA, the transport of amino acids T—RNA, R—RNA (ribosomal).
DNA — double helix, nitrogen bases; adenine—thymine, guanine-cytosine, sugar-deoxyribose. Responsible for storing inherited information.
RNA is a single helix, with nitrogen bases: adenine—uracil, guanine-cytosine, sugar-ribose.
Macromolecules of DNA and RNA are giant carbohydrate-phosphate chains with a "fringe" of nitrogenous bases attached to them. The constant composition and complementarity of the chains of the DNA molecule in its helix determines its unique and fundamental biochemical role — to be the Keeper of the entire set of genetic information in each cell and organism as a whole. It should be known that the composition of DNA is constant for all cells of a given organism at any age and under any physiological conditions. It is markedly different for different species. The sequence and number of nitrogenous bases in the polynucleotide chain is also individual for each organism.
Experience
Students conduct experiments with pure polymers in order to determine the polymer-based plastics and fibers they are given in the next lesson.
Sequence of operations:
1. Describe the external pitchfork of the polymer.
2. Relation to heating (softens, becomes transparent, melts, can the thread be pulled or not, turns black, etc.).
3. polymer Combustion (flame color, characteristic signs of combustion — Smoking flame, intermittent flame, characteristic smell, whether burning outside the flame).
4. Determination of decomposition products (indicator action), discoloration of bromine or iodine water solution).
5. for fabrics, check after burning whether the remaining lump is rubbed.
Results
Polyethylene![]() -greasy
to the touch, easily softens when heated, becomes transparent, stretches into a
thread, it is fragile, burns with a bluish flame, the smell of burning candles
(paraffin) spreads, it burns outside the flame.
-greasy
to the touch, easily softens when heated, becomes transparent, stretches into a
thread, it is fragile, burns with a bluish flame, the smell of burning candles
(paraffin) spreads, it burns outside the flame.
Polystyrene 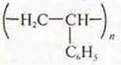 -
brittle, when heated, softens, stretches into a thread, burns with a Smoking
flame, outside the flame burns, the smell of styrene spreads, discolors the
solution of VG2(bromine) or I2(iodine); there is a light
depolymerization.
-
brittle, when heated, softens, stretches into a thread, burns with a Smoking
flame, outside the flame burns, the smell of styrene spreads, discolors the
solution of VG2(bromine) or I2(iodine); there is a light
depolymerization.
Polyvinyl
chloride 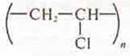 —
softens at low temperatures burns with a smoky flame, does not burn outside the
flame, decomposes with the release of hydrogen peroxide; universal indicator,
changes color to pink.
—
softens at low temperatures burns with a smoky flame, does not burn outside the
flame, decomposes with the release of hydrogen peroxide; universal indicator,
changes color to pink.
Polymethyl-methacrylate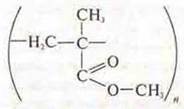 -softens,
burns with a slight crackling, blue flame, spreads the smell of ether, burns
outside the flame.
-softens,
burns with a slight crackling, blue flame, spreads the smell of ether, burns
outside the flame.
Kapron 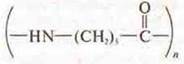 — melts,
forms a hard shiny ball, smells unpleasant, stretches into a thread, burns
outside the flame.
— melts,
forms a hard shiny ball, smells unpleasant, stretches into a thread, burns
outside the flame.
Cotton (S6N10About5)n-burns quickly, the smell of burnt paper, the ash is ground to powder.
Wool![]() -burns
quickly, the smell of burnt feathers, ashes are ground into powder.
-burns
quickly, the smell of burnt feathers, ashes are ground into powder.
Acetate
fiber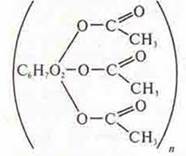 -melts at first, and then
burns quickly, the smell is unpleasant, the ball does not give in to rubbing.
-melts at first, and then
burns quickly, the smell is unpleasant, the ball does not give in to rubbing.
Lavsan 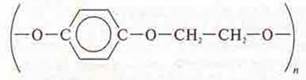 — melts, burns quickly, it is a polyester fiber, the ball is
rubbed, but not completely.
— melts, burns quickly, it is a polyester fiber, the ball is
rubbed, but not completely.
III. Summarizing the lesson results
IV. Homework assignment
§ 10, prepare for practical work.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.