
Factors affecting the speed of chemical reactions - CHEMICAL REACTIONS - LESSON PLANS for CHEMISTRY class 11 - lesson plans - lesson plans-author's lessons-lesson plan-lesson summary - chemistry
The purpose of the lesson: to teach you to explain the influence of various factors on the speed of chemical reactions based on the concept of activation energy; to solve computational problems of this topic.
Basic concepts: velocity constant, acting mass law, temperature coefficient, catalyst, and catalysis: homogeneous and heterogeneous.
Equipment: reagents for demonstration experiments: a) Na2SO4and ВаСl2; b) Mg and HCl; Zn and Hcl; C) Na and N2O; Na and alcohol; d) Mg and Hcl d) h and CH3COOH; e) HCl, Zn (pellets.), Zn (powder); W) H2SO4(p), sodium thiosulfate; S and O2; CACO3(chalk), CACO3(chopped.); h) raw potatoes, boiled (or meat), alcohol, sugars, ash.
Lesson progress
I. Front-end survey
1. To define the speed of homogeneous reaction. Formula for the speed of a homogeneous reaction.
2. To define the speed of heterogeneous reactions. Formula for the rate of heterogeneous reaction.
3. What is the activation energy (Ea)? Why is it important to know theаamount of substances?
4. Calculation task:
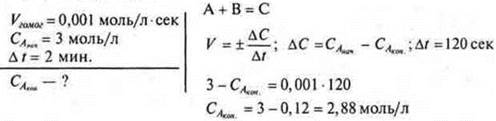
Answer: CA kom= 2.88 mol/l
II. Learning new material
Plan of presentation
1. Factors affecting the rate of chemical reaction.
a) the nature of the reactants,
b) the concentration of the reactants: pressure (for gases)
C) the contact surface of reactants.
d) temperature.
e) catalyst, inhibitors.
2. Solving calculation problems.
The speed of the chemical reaction depends on many factors. The main ones are the nature and concentration of reactants, pressure — if the reaction involves gaseous substances, the contact surface of reactants, temperature, and the action of the catalyst.
We need to consider the influence of each of these factors on the rate of chemical reaction, confirming experimentally.
The first factor — the nature of reacting substances and activation energy are interrelated. If theа ion Density is 40 kJ/mol, this means that most particle collisions result in their rapid interaction. Ion exchange reactions occur instantly, the rate of such reactions is very high, because the reactions involve anions and cations, whichаhave a very small E and A.
If Eais very large, > 120 kJ/mol, this means that only a small number of collisions of interacting particles leads to the formation of new substances. The rate of such a reaction is very small. For example, the interaction of nitrogen with hydrogen to form ammonia is not possible under normal conditions. If Eahas an intermediate value of 40 < EaExamples of such reactions are the interaction of metals with acids, the hydrolysis of sucrose, the interaction of sodium with water, alcohol, and so on.
Thus, the nature of the reacting substances should be understood as:
a) Features of the structure of atoms of elements of metals and non-metals.
Example. K is more active than Na; Mg is more active than Zn, since the radii of the atoms are different, the reducing capacity is different, and the Eand a differ.
Comparative experiment of K and Na interaction with water:
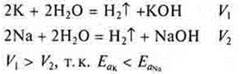
Interaction of Mg and Zn with hydrochloric acid:
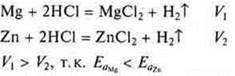
b) Features of chemical bond: types of bond; σ-or π-bond; multiplicity of bond, bond length.
Example. In the nitrogen molecule, N2is a covalent nonpolar bond, the multiplicity of the bond is three; one σ-bond and 2π-bond, the bond is short; EandN2is very high.
In compounds with an ionic bond, eais very small for ions. Reactions are instantaneous.
Experience: ![]()
![]() —
precipitates white instantly.
—
precipitates white instantly.
C) the mutual influence of atoms and groups of atoms is very important in compounds. In the ethanol molecule, the mobility of hydrogen in the group —IT is influenced by a hydrocarbon radical, which slows the reaction rate, if you compare the interaction of sodium in water and sodium with alcohol.
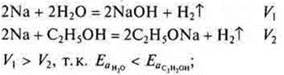
d) If electrons are involved, then the strength of the electrolyte affects the reaction rate. Compare the reaction rate of magnesium with hydrochloric acid-strong acid and magnesium with acetic acid-weak acid.
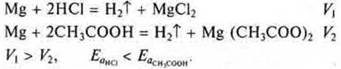
The second factor is the concentration of reactants. In order for the chemical interaction between some particles to take place, they must collide effectively. The greater the concentration of reactants, the greater the number of collisions and, consequently, the higher the reaction rate. On the basis of experiments conducted in 1867 by the Norwegians K. Guldberg and P. In 1865, the basic law of chemical kinetics was formed by the Russian scientist N. I. Beketov and independently of them, and the dependence of the reaction rate on the concentration of reacting substances was established.
The rate of chemical reaction is proportional to the product of the concentrations of reacting substances taken in degrees equal to their coefficients in the reaction equation.
This law is also called the law of active masses (at the end of the XIX century, the term "concentration" was not yet introduced, and chemists used the term "active masses" instead).
For the reaction A + B = D, it is calculated
![]()
and the reaction rate A + 2B = D is defined as
![]()
where CA, CB— concentrations of reactants, mol/l, k1, k2-reaction rate constants.
The velocity constant depends only on temperature and does not depend on the concentration of substances. The velocity constant at constant temperature is numerically equal to the reaction rate, in which the concentration of reactants is equal to 1 mol/L. The law of active masses does not take into account the concentration of reacting substances in the solid state, because they react on surfaces and their concentrations are constant.
Example: ![]()
V = k · WithO2, the reaction rate is proportional only to the oxygen concentration. Thus, the law of active masses takes into account only the concentrations of gaseous or dissolved substances.
Example:
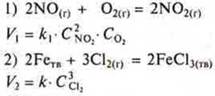
A change in pressure due to the participation of gaseous substances in the reaction also leads to a change in the concentration of these substances. Thus, when the pressure increases by 2, 3 or more times, the volume decreases, and the concentration of substances per unit volume increases by the same number of times, and Vice versa, when the pressure decreases, the volume increases, and the concentration per unit volume will also decrease.
Example:
a
—![]() -
without changing the pressure
-
without changing the pressure
![]()
b) the pressure is increased 3 times,
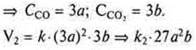
In General, the reaction rate is increased:
![]() - in
27 times;
- in
27 times;
C) the pressure was reduced by 2 times.
![]()
![]() - the
reaction speed decreases by 8 times.
- the
reaction speed decreases by 8 times.
The third factor is the contact surface of the reactants. Reactions take place on the surface — these are heterogeneous reactions.
Speed
formula![]() the
smaller the unit of area S, the greater the reaction speed.
the
smaller the unit of area S, the greater the reaction speed.
Solid reactants should be crushed, ground, i.e. break the structure of the crystal lattice, because particles on a microcrystal are more reactive than the same particles on a "smooth surface".
Experience. To compare the rate of reaction (via overhead projector)
In industry, a "fluidized bed" is used to conduct heterogeneous reactions, increasing the contact surface of reacting substances and removing the reaction products. In the production of sulfuric acid, pyrrolidane is fired in the "fluidized bed"; in organic chemistry, catalytic cracking of petroleum products and catalyst regeneration are carried out in the" fluidized bed".
The fourth factor is temperature.
The higher the temperature, the more active particles, as E increases,butincreases their speed, which leads to a large number of collisions at the interaction with each other. There is an increase in the reaction speed. Reaction between oxygen and hydrogen at t° = 20 °C for 54 billion cubic meters. years pass by 15%, at 500 °C - in 50 minutes, at 700 °C-instantly.
In
1844, J. Vanth-Hoff found that when the temperature rises for every 10°, the
rate of chemical reaction increases by 2-4 times. This value is called the
temperature coefficient of the reaction, denoted y-gamma. For each reaction
with a number from 2 to 4. when the temperature rises from t°1to t°2,
the reaction accelerates![]() several
times, i.e. the reaction rate at
several
times, i.e. the reaction rate at ![]()
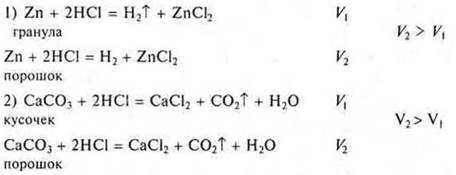
Experience. Interaction of Hcl with zinc under: a) normal conditions (V1); b) when heated (V2). V2 > V1.
For more precise calculations use the Arrhenius equation:
![]()
where K — the rate constant; Z — the number of collisions of molecules, particles per second per unit volume; R — stereochemistry of the multiplier; e — base of natural logarithm (e = 3,718); R— universal gas constant (R = 8,314 j/Molk); T — temperature in Kelvin (273° + t°C); Eais the activation energy.
However, increasing the temperature is not always applicable, because the raw materials can start to decompose, evaporate, solvents, or the substances themselves evaporate.
Experience 1. Interaction of sodium thiosulfate Na2S2O3with sulfuric acid:
a) at room temperature;
b) when the temperature rises by 10° above room temperature;
C) when the temperature rises by 20° above room temperature.
For each stage, determine the time of turbidity, precipitation of sulfur, we note ta> t>b> t>вC .
![]()
Experience 2. It is possible to show the burning of sulphur in air and in pure oxygen. Pre-collect the oxygen in the flask (either by decomposing Cmpo4, or by decomposing H2O2on the catalyst).
The bright glow — blue flame when burning sulfur in pure oxygen is due to the fact that in this case the reaction rate will be higher, because the concentration of oxygen is high, and in the air it is about 20 %.
The fifth factor is a catalyst, an inhibitor. Catalysts are substances that reduce the reaction mechanism and direct it along the energy path in a more profitable way, with a lower activation energy.
Catalysts are substances that participate in a chemical reaction and change its speed or direction, while remaining unchanged qualitatively and quantitatively at the end of the reaction. If the catalyst and reagents are in the same aggregate state — it is a homogeneous catalysis.
Example.
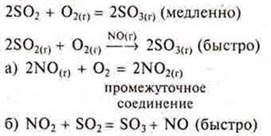
If the catalyst and reagents are in different aggregate States — this is heterogeneous catalysis.
![]()
The mechanism of action of the catalyst is explained by the formation of intermediate compounds, which then easily enter into a chemical reaction.
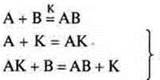 they
flow at a high speed
they
flow at a high speed
AK-intermediate connection.
In the presence of a catalyst, the path along which the total reaction takes place changes, so its speed changes. Positive catalysts increase the reaction rate.
Negative catalysts — inhibitors-slow down the speed of the chemical reaction. They react at a high rate with active particles and form low-activity compounds. The rate of reaction slows down, and the chemical reaction stops. Inhibitors stabilize acids when they are transported in steel containers, suppress the action of hydrogen peroxide, and monomers prevent premature polymerization.
Catalysts and inhibitors work very effectively in a living organism. Catalysts in a living organism are called enzymes. Thanks to them, many complex reactions take place in a living organism at a low temperature with high speed. Enzymes differ in their specificity, each of them accelerates only one chemical reaction with a yield of 100 %. Inhibitors in a living organism suppress various harmful oxidation reactions in tissue cells that can be initiated by radioactive radiation.
Experience.
1) a Piece of sugar quickly ignites if ash is applied to it (in ash, Li+is the catalyst).
2) the Enzyme catalase. the effect of catalase on hydrogen peroxide.
In test tubes put a piece of raw potatoes and boiled, add hydrogen peroxide. The rate of elimination reaction of O2, where raw potatoes, is greater than with boiled potatoes. Raw potato-catalase-enzyme is not destroyed.
You can use a piece of meat-cooked and raw (for comparison).
Thus, to increase the rate of chemical reaction, factors must be taken into account: the nature of the reacting substances, the contact surface, concentration, temperature, and catalysts.
III. Solving calculation problems
Task 1. Write mathematical expressions for the rates of reactions occurring according to the equations:
![]()
Task
2. How will the reaction rate change![]() if the
concentration of hydrogen is increased by 2 times?
if the
concentration of hydrogen is increased by 2 times?
![]() —
before increasing the concentration of hydrogen.
—
before increasing the concentration of hydrogen.
![]() —
with an increase in the concentration of hydrogen.
—
with an increase in the concentration of hydrogen.
![]()
The reaction rate (2) increased by 4 times.
Task
3. How will the reaction rate change![]() if the
pressure is reduced by 100 times?
if the
pressure is reduced by 100 times?
![]() When
the pressure is reduced by 100 times the concentrations of H2and CL2
decrease by 100 times⇒
When
the pressure is reduced by 100 times the concentrations of H2and CL2
decrease by 100 times⇒
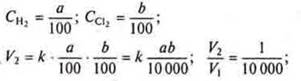
⇒ the reaction speed will decrease by 10,000 times.
Task 4. How many times will the reaction rate increase when the temperature rises from 50° to 100°C, if the temperature coefficient of the speed is equal to 2?
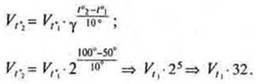
The reaction speed will increase by a factor of 32.
Task 5. Determine the temperature coefficient of the reaction rate, if when the temperature rises from 10° to 50°, the reaction rate increased by 16 times.
![]()
Answer: γ = 2.
III. Homework assignment
§ 13. Tasks # 1-5, verbally 6-10.
Be prepared to work independently on all assignments.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.