
Lesson-lecture on "Hydrolysis of inorganic substances-salts" - DISPERSED SYSTEMS. SOLUTIONS. PROCESSES OCCURRING in SOLUTIONS - LESSON DEVELOPMENTS in CHEMISTRY grade 11 - lesson developments-lesson developments - author's lessons - lesson plan-lesson summary - chemistry
Lesson objectives: to form an idea about the hydrolysis of the essence of hydrolysis of salts; learn how to draw up equations of reactions of hydrolysis in ionic and molecular form, determine the reaction type and the environment of the electrolyte based on the salt composition.
Basic concepts: hydrolysis, hydrolysis by cation, hydrolysis by anion, molecular form of the hydrolysis equation, General ionic and short ionic types of the equation, reaction of the medium.
Equipment: H2O (distil.), alCL 3(CR.), Na2CO3(TV.), NaCl, CuCl2, universal indicators, alcohol lamps, test tubes, holders.
Lesson progress
I. Organizational moment
Setting goals and tasks for the lesson. When studying the topic of "Hydrolysis", it is very important to know the concepts of "hydrogen indicator", "types of shektrolite medium шектролита".
Plan of presentation
1. Experiment. Determination of the reaction of the medium of salt solutions by a universal indicator.
2. The term "hydrolysis of salts". Algorithm for calculating the equation of salt hydrolysis:
a) by cation;
b) by the anion.
Conditions for strengthening and weakening of hydrolysis.
3. Hydrolysis of salts formed by weak acid and weak base.
4. the Significance of salt hydrolysis in a living organism, in nature, in everyday life.
II. Learning new material
Experiment:
Determination of the reaction of the medium of electrolytes — solutions of salts by a universal indicator.
AlCl33 pink color pH < 7 среда кислая
Na2CO3 blue pH > 7 medium alkaline
NaCl green pH = 7 medium neutral
It is necessary to scientifically substantiate the current phenomena.
Hydrolysis is the interaction of salt with water, resulting in the formation of a weak electrolyte. If the acid is an acidic salt, if the base is the main salt, and the solution medium changes. Hydrolysis is a reversible process. The hydrolysis is subjected to a soluble salt, comprising either cations of a weak electrolyte, or the anion of a weak electrolyte. If the cation is a weak electrolyte, it is hydrolyzed by the cation. If the anion is a weak electrolyte, it is hydrolyzed by the anion. If the cation and anion are multicharged, hydrolysis proceeds stepwise. If the salt consists of a cation and an anion of weak electrolytes, irreversible hydrolysis takes place. Salts formed by cations and anions of strong electrolytes, as well as insoluble salts in water, do not undergo hydrolysis.
Example:
In the experiment there were salts:
AlCl33-the salt is formed by a weak electrolyte-base Al(OH)3, strong acid-hydrochloric, hydrolyzed by cation.
Na2CO3-the salt is formed by a strong base NaOH and a weak acid H2CO3-carbonic, hydrolyzed by the anion.
The NaCl salt is formed by a strong base NaOH and a strong acid HCl, there is no hydrolysis.
The algorithm of drawing up of the equation of hydrolysis of salt:
1. Make an equation of salt dissociation, determine the ion of a weak electrolyte.
2. Make an equation of its interaction with water, determine the products of hydrolysis in the form of ions.
3. make a conclusion about the electrolyte medium.
4. Make an equation in molecular and ionic form.
![]()
Al3+- aluminum cation, weak base of hydrolysis by cation
![]()
the environment is acidic, because [N+] > [HE>-]
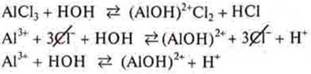
Another variant of the algorithm for composing the salt hydrolysis equation:
a) according to the chemical formula of the salt, determine what acid and what base the salt is formed of;
b) write the left side of the equation in molecular form;
C) make an equation in General ionic form, let's assume, according to this equation, the products of the right side of the equation in molecular form;
d) reduce the same ions in the left and right parts of the equation of the General ionic form;
e) compose the hydrolysis equation in a short form, determine the medium.
a) Na2CO3-salt formed NaOH-strong base, H2CO3-weak acid, hydrolysis by anion CO32-;
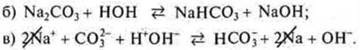
We assume the products of the right part of the equation: nahco3 saltand NaOH base; we write them in the right part of the molecular equation;
d) make a short ionic equation of hydrolysis, reduce sodium cations:
![]()
Conclusion: [ON-] > [N>+] - the medium is alkaline; pH > 7.
Let's make a General conclusion of the experiment conducted at the beginning of the lesson: the salts were subjected to hydrolysis, so the solution caused a certain reaction of the medium.
If you want to give the answer quickly, without making up the equation of salt hydrolysis, you should remember: "the strong overpowers the weak." To determine what power of electrolytes formed salt, if a strong base — alkali reaction medium: Tod core> Forkeys d, if a strong acid — acidic medium: Tod sour> Tod coreif and base, and strong acid reaction of the medium neutral: Tod sour= Tod core.
Example. What reaction of the medium do solutions of salts CuSO4, KNO2, Na2SO4have ?
CuSO4- the reaction of the medium is acidic, since the salt is formed by a strong acid-sulfuric.
KNO2-the reaction of the medium is alkaline, since the salt is formed by a strong base.
Na2SO4- the reaction of the medium is neutral, because the salt is formed by a strong acid and a strong base.
If the salt is formed by a multicharged ion, then hydrolysis proceeds stepwise.
Example:
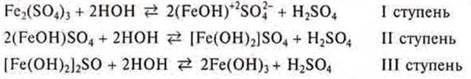
Why is there no precipitation during salt hydrolysis, i.e. hydrolysis does not reach stage III? Since hydrolysis is a reversible process, as soon as the concentration of hydrogen cations begins to increase, then according to the principle of Le Chatelier, the equilibrium shifts towards the reverse reaction, towards the initial products.
If you want to increase the hydrolysis, you can increase the t°, increase the concentration of starting products, or add acid or hydrogen cations to the solution. Hydrolysis will be suppressed.
To enhance the hydrolysis of the salt, you should add a compound that binds the hydrogen cation to the solution, i.e. add an alkali solution. Observed the formation of water H++ ON-= H2O, as a result of which the concentration of hydrogen cations decreases and the equilibrium shifts towards a direct reaction. Hydrolysis is enhanced. You can юбавитьdilute the salt solution, which has an alkaline reaction of the medium.
In the case of hydrolysis of a salt formed by a weak acid, a weak base, the final products are formed — a weak base, a weak acid. Hydrolysis is irreversible.
![]()
What reaction of the medium is possible with such hydrolysis? The medium is determined by comparisonдof weak electrolytes To d. The environment is defined bya large KD value.
Example. ![]()
![]()
In this case, the reaction of the medium will be neutral, becausethe Kvalues of the reagents are equal.
In the solubility table of salts, acids, bases in water, the note indicates "decompose in the aquatic environment", i.e. undergo hydrolysis.
III. Summary and conclusions
1. Hydrolysis — the interaction of salt with water to form a weak electrolyte and change the reaction of the medium.
2. Hydrolysis is a reversible process.
3. hydrolysis is Possible: a) by cation; b) by anion.
4. the reaction of the medium depends on the ratio Todof the electrolytes that formed the salt.
5. Hydrolysis is irreversible if at least one of the products of hydrolysis leaves the sphere of the reaction. It immediately goes through both the cation and the anion.
Hydrolysis is of great importance in a living organism, in living nature, and in the practical life of a person.
As a detergent in ancient times used ash, which includesK2CO3 is potassium carbonate, which is hydrolyzed in water to form an alkaline reaction. The solution becomes soapy. Currently, soap, washing powders-sodium, potassium salts of higher fatty carboxylic acids — stearic and palmitic-are used in everyday life.
Hydrolyzing in an aqueous solution they give an alkaline reaction:
![]()
In the composition of cleaningmedia BXinclude salts of inorganic acids: phosphate, carbonate, they enhance the washing effect.
In the photographic business of salt-borax Na2B2O4, Na2CO3, K2CO3, hydrolyzing, creates an alkaline reaction.
With a lack of soil acidity, plants develop the disease chlorosis. Fertilizer is introduced into the soil (NH4)2SO4, which increases soil acidity due to cation hydrolysis:
![]()
Thanks to the salts that are part of the blood-Nahco3,NO 2, NPO4, a certain reaction of the medium is maintained. They regulate excess H+and excess OH-. With an excess of H+, they bind toOH-and the balance shifts to the right, increasing hydrolysis.
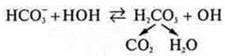
If there is an excess ofO-, the equilibrium will mix to the left. Thanks to this, the pH of blood fluctuates slightly.
A specific environment is maintained in the oral cavity. Thanks to the anion HPO42 -, which is part of saliva, the pH ranges from 7 to 7.5.
IV. Homework assignment
Section 16, p. 163-173, № 1, 3, 4, 5, 6.
Independently analyze the hydrolysis of other inorganic compounds-p. 173-174 (summary in the notebook).
Repeat esters, proteins, and carbohydrates.
Fastening
Execute # 6 § 16 p. 174.
|
KI |
AlCl3 |
K2SO3 |
(NH4)2SO4 |
NaCN |
|
|
Medium |
pH = 7 Neut. |
pH < 7.5 . < 7 кислno problem . |
pH > 7> щелslot . |
pH < 7.5 . < 7 кислno problem . |
pH > 7> щелslot . |
|
The power of El-TA |
is Strong. mainfeatures Strong. кислno problem . |
Weak. aboutSN. Strong. кислno problem . |
Strong. mainfeatures Weak. кunfortunately. |
Weak. aboutSN. Strong. кислno problem . |
Strong. mainfeatures CIL. mainfeatures |
|
Fanapt-n |
- |
- |
crimson. |
" |
Malin," I said. |
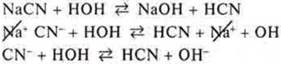
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.