
Practical work # 3 " Solving experimental problems on the topic "Hydrolysis.Ion exchangereagents "" - DISPERSED SYSTEMS. SOLUTIONS. PROCESSES OCCURRING in SOLUTIONS - LESSON DEVELOPMENTS in CHEMISTRY grade 11 - lesson developments-lesson developments - author's lessons - lesson plan-lesson summary - chemistry
Objectives of the lesson: to improve skills in performing a chemical experiment, observing the rules of OT and TB; to consolidate the ability to confirm theoretical knowledge with a chemical experiment.
Equipment: a set of reagents for each table (where there are acids, alkalis, salts, indicators); assignment of practical work in two variants.
Lesson progress
I. Organizational moment
Instructing students on the theoretical work, TB and FROM performing the work.
II. Performing work on options
|
Option I |
Option II |
|
1. To conduct an experiment between solutions of electrolytes. Mark the observed phenomena. Give a reasonable answer. |
|
|
a) sodium carbonate and sulfuric acid. b) sodium hydroxide and zinc sulfate. |
a) barium chloride and iron sulfate (II) b) potassium carbonate and hydrochloric acid |
|
Equations of reactions are made in a molecular General and brief ionic form. |
|
|
2. Examine the salt solutions with indicators. |
|
|
a) zinc sulfate. b) potassium nitrate. C) sodium carbonate. |
a) sodium sulfate b) iron(III) chloride) C) potassium sulfite |
|
Explain the observed phenomena. Write the equation of hydrolysis in ionic and molecular forms. |
|
|
3. What happens when electrolyte solutions are drained? |
|
|
Aluminum chloride and sodium carbonate. When приливанииexcess alCL 3or excess Na2CO3 flows in |
A zinc pellet was placed in a solution of zinc chloride. If приливании HCl or excess ZnCl2 is present |
III. Homework assignment
To prepare for the control work № 3 on the topic "Chemical reactions. Disperse system. Processes occurring in solutions".
Answers to practical work tasks
Option I
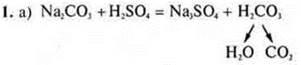
Gas and water release is observed.
![]()
The ion exchange reaction goes to the end, because gas and iodine are formed — a weak electrolyte.
![]()
There is a precipitation of gelatinous, white, translucent sediment.
![]()
The reaction of ion exchange goes to the end, because a precipitate is formed, a very weak electrolyte.
2. The result of observations:
|
ZnSO4 |
KNO3 |
Na2CO3 |
|
|
Base |
Weak |
Strong |
Strong |
|
Acid |
Strong |
Strong |
Weak |
|
pH |
< 7 |
7 |
> 7> |
|
Wednesday |
Acidic Environment |
Neutral |
Alkaline |
|
Universal indicator |
Pink |
Green |
Blue |
|
Phenolphthalein |
— |
- |
Crimson |
|
Litmus blue |
Red |
Purple |
Blue |
|
Methylorange |
Pink-red |
Orange |
Yellow |
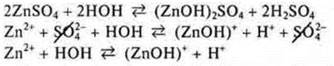
![]() the environment
is acidic, the hydrolysis of the cation
the environment
is acidic, the hydrolysis of the cation
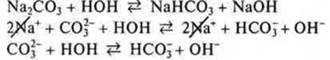
![]() the
environment is alkaline, the hydrolysis of the anion
the
environment is alkaline, the hydrolysis of the anion
KNO3-hydrolysis is not present, because the salt is formed by a strong base and a strong acid.
3. When merging solutions АlСl3and Na2CO3there is a co-hydrolysis.
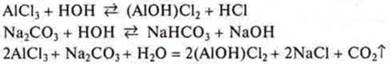
Gas is released if we take AlCl3 in excess3; if we take Na2CO3 in excess2CO3, then gas and sediment are observed.
![]()
Option II
![]() white
sediment will fall out
white
sediment will fall out
![]()
The reaction of ion exchange goes to the end, because it forms a precipitate,
![]() gas
and water release is observed
gas
and water release is observed
![]()
The ion exchange reaction goes to the end, because gas and water are formed.
2. The result of observations:
|
Na2SOl |
FeCI3 |
K2SO3 |
|
|
Base |
Strong |
Weak |
Strong |
|
Acid |
Strong |
Strong |
Weak |
|
pH |
7 |
< 7 |
> 7> |
|
Wednesday |
Neutral |
Acid |
Alkaline |
|
Universal indicator |
Green |
Pink |
Blue |
|
Phenolphthalein |
— |
- |
Crimson |
|
Blue litmus |
Purple |
Red |
Blue |
|
Methylorange |
Orange |
Pink-red |
Yellow |
Na2SO4-no hydrolysis, the salt is formed by a strong base and a strong acid.
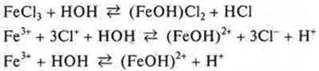
![]() acidic
environment, the hydrolysis of the cation.
acidic
environment, the hydrolysis of the cation.

![]() the
environment is alkaline, the hydrolysis of the anion.
the
environment is alkaline, the hydrolysis of the anion.
![]()
When adding Zn, the medium is acidic, and hydrogen is released.
![]()
When HCl is added, zncl2 hydrolysis is weakened and the rate of gas release decreases. When ZnCl2 is added, hydrolysis is enhanced, and the rate of gas release increases.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.