
Classification of inorganic substances-SUBSTANCES AND their PROPERTIES - LESSON PLANS for CHEMISTRY 11 class - lesson plans-lesson plans-author's lessons-plan-lesson summary - chemistry
Lesson objectives: to summarize and consolidate the knowledge on the classification of inorganic substances; to teach on the basis of the composition of the molecules of the substance properly called substances; to consider the relationship and interdependence of composition, structure and properties of matter; to give an idea of the value of inorganic substances in daily life; to give the notion of complex compounds.
Basic concepts: simple substances — metals, nonmetals; complex substances — oxides, acids, salts; hydroxides-bases, hydroxides-acids, amphotericity; double and complex salts; ion-complexer, ligatun, inner sphere, outer sphere, coordination number.
Equipment: collection "Metals", "non-Metals" - sulfur, graphite; models of types of crystal lattices, table; VG2, I2, red phosphorus; oxides: Fe2O3, CuO, Al2O3, Cao, SG2O3; acids: H2SO4, HCl, HNO3; alkalis: NaOH, KOH, Na2CO3NaHCO3, (CuOH)2CO3, KMnO4, NH3· H2O, CuSO4; collection "Minerals and rocks"; test tubes.
Lesson progress
I. Organizational moment
The teacher analyzes the result of performing test work # 3, focuses the attention of students on the mistakes made in the work, explains the correct performance of some tasks. It offers students to work on mistakes and offers individual tasks to improve their score.
Then the teacher introduces students to the new topic "Substances and their properties", as well as to the lesson topic: "Classification of inorganic substances".
II. Learning new material
The material is familiar, but it was studied in grades 8 and 9. Lot of things are forgotten. It is necessary, working with the text of the textbook, § 17, to remember the composition, classification, nomenclature of inorganic substances.
Plan of presentation
1. Determination of simple and complex substances.
2. Classification of simple substances: metals and non-metals. Structure of atoms, types of chemical bonds, types of crystal lattices, features of properties (drawing up a table).
3. Classification of complex substances; oxides, hydroxides: acids, bases; salts.
4. Characteristics of a complex substance according to the plan: definition, classification, nomenclature, structure (chemical bond, crystal lattice), physical properties, meaning in everyday life.
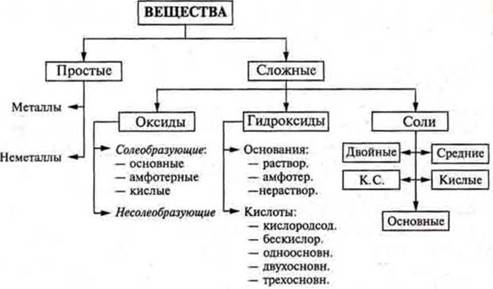
Based on the composition, all substances are divided into simple and complex ones.
Let's recall the definition of simple and complex substances.
Simple substances-molecules consist of atoms of the same kind.
Example: oxygen, sulfur, nitrogen.
Complex substances-molecules are made up of atoms of different types.
Example: water, carbon monoxide (N2), sulfuric acid, etc.
All simple substances are divided into metals and nonmetals based on the structure of atoms, the type of chemical bond, the type of crystal lattices, and their physical and chemical properties.
Students make a table in their notebooks:
|
Substance |
Structure of an atom |
Type of chemical bond |
Structure of simple substances, type of crystal lattice |
Properties of the substance |
|
All metals
All non-metals
are Noble gases |
Small number of electrons at the external energy level (less than 3)
A large number of electrons at the external energy level (more than 4)
The external energy level is complete (an octet of electrons or 2 u is Not) |
Metal bond
Covalent polar bond
There are no connections between the atoms |
Metal crystal lattice
Molecular or atomic crystal lattice
Molecular crystal lattice |
Reducing properties
Redox properties
Inert |
Students examine the collection of metals and some non-metals that are available in the classroom. Recall the types of crystal lattices, considering their models and the table (atomic, molecular, metallic).
What is the classification of complex substances?
Complex substance:
a) oxides; b) bases; C) acids; d) salts.
Generalizing and a more comprehensive classification of inorganic substances is presented in the textbook § 17 p. 179, scheme 7.
In notebooks, you should, according to this classification, make notes on the following plan:
1. The class definition, General formula.
2. Classifications; name.
3. Signs (chemical connection) of the substance structure, physical properties.
4. The value of the substances in everyday life.
Oxides are complex substances formed by two elements, one of which is oxygen: emAboutn.
According to their composition, oxides can be formed by metals: Cao; PBO and non-metals: CO2; NO2.
According to their properties, oxides are salt-forming and non-salt-forming (also called indifferent). Salt-forming substances are divided into basic, acidic and amphoteric ones. The main oxides are formed by metals, they correspond to bases; acid oxides, as a rule, are formed by non-metals and metals in the maximum C. O. they correspond to acids. Amphoteric oxides are formed by transition elements.
Example: barium oxide — Bao, the main oxide, since BA is a typical metal. Sulfur(IV) oxide is SO2, an acidic oxide, since S is a typical nonmetal. Manganese(VII) oxide MP2O7is an acid oxide, since MP is a metal element in the maximum S. O., aluminum oxide-Al2O3is an amphoteric oxide, since Al is a transition element.
The name of the oxide must specify The s. O. if the generating element has several S. o. S.
Example: ![]() — sulfur
oxide (IV);
— sulfur
oxide (IV);![]() — sulfur
oxide (VI).
— sulfur
oxide (VI).
Non-salt-forming oxides are not so much, they should be remembered:
CO-carbon monoxide (II);
NO-nitric oxide (II);
N2O — nitrous oxide(I).
Oxides formed by metals have an ionic bond, while transition metals have a covalent polar bond. Crystal lattices can be ionic or atomic:
the barium oxide — ion in the crystal lattice;
aluminum oxide-atomic crystal lattice;
minerals: corundum, sapphire, ruby.
Oxides formed by non-metals have covalent polar bonds, molecular (carbon dioxide, "dry ice"), atomic (silicon oxide (quartz, rock crystal, agate, etc.) crystal lattices.
The teacher shows students a collection of rocks and minerals, which include oxides of: quartz (SiO2), corundum (Al2O3), asbestos (CaO · 3MgO · 4SiO2), talc (3MgO · 4SiO2·H2O); clay: white, red: includes oxides: Al2O3· PN2O — white; bauxite Fe2O3is included in red clay. Iron ores: Fe2O3— red ironstone, Fe3O4-magnetic ironstone.
The composition of the air includes: carbon monoxide (IV) — CO2, water — H2O, harmful impurities, CO — carbon monoxide, which is formed when the fuel is not fully burned.
Application of oxides:
H2O — water; the most important mineral of the Earth; participates in the cycle of substances;
SiO2-silicon oxide; it is a part of most minerals found in nature: silica, talc, asbestos, Jasper, rock crystal, feldspar;
Fe2O3, Fe3O4-ores for the production of cast iron and steel;
CO2— carbon dioxide; the cycle of substances in nature; photosynthesis;
CO-carbon monoxide, poison; it is formed when the fuel is not fully burned.
All oxides correspond to hydroxides-compounds consisting of three elements, two of which are hydrogen and oxygen.
Depending on the nature of the oxide, the hydroxide formed by it can be a base or an acid. The main oxides correspond to base hydroxides. Example, WAO-WA(ON)2.
Bases — complex substances, consisting of atoms of metal and one or more hydrocorp (—HE):
![]()
According to the solubility in water, bases are well-soluble in water-alkalis (KOH, NaOH), poorly soluble in water (CA(OH)2), insoluble in water (si (OH)2).
The bases have an ionic bond between the metal and the hydroxo group, and in the hydroxo group there is a covalent polar bond. The crystal lattice is ionic, solid.
A special group of bases are insoluble in water, which correspond to amphoteric oxides of S. O. +2, +3, +4.Such bases are also called amphoteric. They exhibit acid-base properties.
Example:
![]()
For them, covalent polar bonds and molecular crystal lattices are possible.
Scope of application of bases:
NaOH-sodium hydroxide, "caustic soda"; refining of petroleum products, bleaching of paper, soap production, drying of gases in organic synthesis;
Ca (HE)2-calcium hydroxide, stone lime; mixed with sand lime solution, whitewash; sucrose production;
NH3· H2O-hydrate of ammonia, ammonia alcohol-medicine, ammonia water — liquid nitrogen fertilizer;
Al(HE)3-aluminum oxide; medicine-Almagel, a drug with an enveloping adsorbing effect.
![]() —
becoming a laboratory assistant;
—
becoming a laboratory assistant;
Fe(HE)3 — iron (III) hydroxide-component of yellow pigment for paints and enamels, absorption mass for natural gas purification; catalyst in organic synthesis.
Acidic oxides, hydroxides correspond to the acid.
![]() —
General formula of acids.
—
General formula of acids.
![]() —
acid residue (acid (English) - acid).
—
acid residue (acid (English) - acid).
In the presence of oxygen in the molecule are oxygen-containing acid (H2SO4, HNО3), anoxic (HCl, H2S); the number of hydrogen atoms in the molecule, the monobasic acids are HCl, HNO3; dibasic — N2FROM3, H2S; tribasic — N3RO4.
The chemical bond between atoms in acids is covalent polar. The structure of substances is molecular.
The use of acids:
H2SO4-sulfuric acid; production of mineral fertilizers, aphids of oxygen-free acids; cleaning of petroleum products, metal surfaces; organic synthesis: production of fibers, paints, lacquers, medicines; explosives; filling of batteries;
HNO33-nitric acid; production of nitrogen fertilizers, medicines; organic synthesis; rocket fuel oxidizer;
N3RHO4-phosphoric acid; production of phosphoric fertilizers;
HCl-hydrochloric acid; metal etching, Dormouse production, food industry, medicine, organic synthesis.
A large class of complex substances are salts.
Salts are complex substances consisting of metal cations and acid residue anions (this is the General definition).
There are salts: a) medium; b) acidic; C) basic; d) double; e) complex.
Medium salts are formed when hydrogen atoms in an acid molecule are completely replaced with metal atoms or when hydroxogroups in base molecules are completely replaced with acid residues:
N3RO4- Na3RO4; VA(ON)2-Vasl2;
H2SО4 - K2SО4; Fe(OH)3 - Fe(NO3)3.
Acidic salts are formed when incomplete substitution of hydrogen atoms in acid molecules for metal atoms:
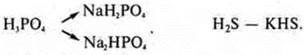
The main salts are formed when the hydrox groups in the bases are not fully replaced with acidic residues:
Fe(OH)3 - (FeOH)Cl2; Cu(OH)2 - (CuOH)NO3.
Double salts and complex salts differ from each other in the nature of dissociation in aqueous solutions. Double salts dissociate in one step into metal cations and anions of acid residues.
Example: double salt KAl(SO4)2:
![]()
Complex salts when dissociated form complex complex ions that are stable in aqueous solutions. Example [Cu(NH3)4]SO4is a complex salt.
![]()
For classes that have an increased level of learning material, you should describe complex compounds in more detail.
The theory of structure of complex compounds was developed by A. Werner (Swiss chemist). According to the theory of A. Werner, in the center of the complex compound is an ion-complexer. It can be a metal (mainly d-elements-having free orbitals), as well as an element that has unshared pairs of electrons.
Around the complexing ion are oppositely charged ions or neutral molecules called ligands (addends). The complexing ion and ligands make up the inner sphere of the complex compound, which is written in square brackets. The number of ligands corresponds to the coordination number of the complexing ion. Most typical:
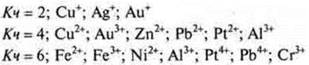
The charge of the inner sphere is equal to the sum of the charges of the complexing ion and the ligand.
Ions that have not entered the inner sphere form the outer sphere.
Experience.
Getting [Cu(NH3)4]SO4.
CuSO4 + 4NH3 = [Cu(NH3)4]SO4-dark blue solution.
Classification of complex connections, but the charge of the inner sphere:
a) cationic complexes:
[Cu(NH3)4]2++ SO42 -the name is made up starting with the anion of the molecule; the complexing ion is named in Russian in the genitive case:
Copper (II) tetraamine sulfate)
b) anionic complexes. The complexing ion is called in Latin with the suffix " at»:
Na2+[Zn(OH)4]-2tetrahydrozoline sodium;
C) neutral complexes. The complexing ion is called in Russian in the nominative case: [Fe(CO)5] Penta-carbonyl-iron.
K+3[Fe+3(CN-)6]-3-anionic complex of hexa-cyono-ferrate (III) of potassium.
[Co(Br)(NH3)5]SO4-sulfate-Penta-amine-bromo-cobalt (III).
Consider the structure of complex compounds (as a confirmation of knowledge about complex compounds).
![]()
[Ag(NH3)2]+— the inner sphere;
Ag+is a complexing ion;
2NH2NH 30-ligands;
CL-— the outer sphere;
Silver (I) diammin chloride.
The importance of complex compounds in nature is enormous. Chlorophyll and hemoglobin are complex compounds of a living cell.
Chlorophyll is a complex compound, the complexing ion is magnesium; chlorophyll is responsible for photosynthesis. Hemoglobin is a complex compound, and iron is the complexing ion. Hemoglobin is responsible for gas exchange in the cell: it supplies oxygen to the cell and removes carbon dioxide. Vitamin B-12 is a complex compound of cobalt. Thus, the metabolism depends on complex compounds in living organisms.
In salts there is an ionic bond, a covalent polar bond, and in complex compounds between the complexing ion and ligands-a bond by the donor-acceptor mechanism.
In everyday life of the salt are of great importance (a demonstration of some salts). At home Nahco3— sodium bicarbonate, baking soda;CaCO3- calcium carbonate, chalk, limestone, marble; Na stearate, K — solid and liquid soap; KMPO4-disinfectant: mineral fertilizers: nitrogen NH4NO3— ammonium nitrate, potash KCl — potassium chloride, phosphorous (NH4)2HPO4is the hydrogen phosphate of ammonium.
In industry: catalyst saltsAlcl 3; FeBr3; biological value: salts NaCl, KCl; Na2HPO4; NaHCO3; CaF2; CA3(PO4)2; malachite (CuOH)2CO3is a mineral.
At the end of the lesson, as a result, you can suggest that you independently make a classification scheme for inorganic substances without a textbook.
III. Homework assignment
§ 17, p. 176-178. Give names and distribute them according to the classification:
S, Al(OH)3; H2SO3; NO; BaO; Р2O5; Hi; Mg(OH)2; CaSO4; H3RO4; NaHSO4; (ZnOH)Cl; KNO3; Li2CO3; Na3[AlF6]; NH4Fe(SO4)2; CaHPO4.
Recommendations for the teacher
In this lesson, students can be asked to prepare abstract reports on simple substances-metals and their compounds, as well as on simple substances — non-metals and their compounds. Presentations will be heard at seminars on this topic.
Message plan
1. The history of the discovery of the element. Why is it so named? Being in nature, receiving.
2. Features of the structure of the atom. Oxidation degree.
3. Features of physical properties of simple substances. Chemical bond, crystal lattice.
4. Features of chemical properties.
5. Critical applications in pure form and in the form of compounds.
Students should be reminded that the abstract message must be shown to the teacher in advance for review and recommendations.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.