
Compounds of non-metals: hydrogen compounds, oxides and hydroxides-SUBSTANCES AND their PROPERTIES-LESSON DEVELOPMENTS in CHEMISTRY 11 class - lesson developments-lesson developments-author's lessons-plan-lesson summary - chemistry
The purpose of the lesson: to teach the correct formula of compounds of non-metals: oxides, their corresponding hydroxides, hydrogen compounds, taking into account their possible S. O. A., as well as to describe their properties and changes depending on the structure of the atom, the position in the PSE D. I. Mendeleev.
Basic concepts: basic character, acidic character, oxidizer, reducing agent, covalent polar bond, bond polarity.
Lesson progress
I. Organizational moment
Setting goals and tasks for the lesson.
II. Checking that homework has been completed correctly
Completing homework at the blackboard.
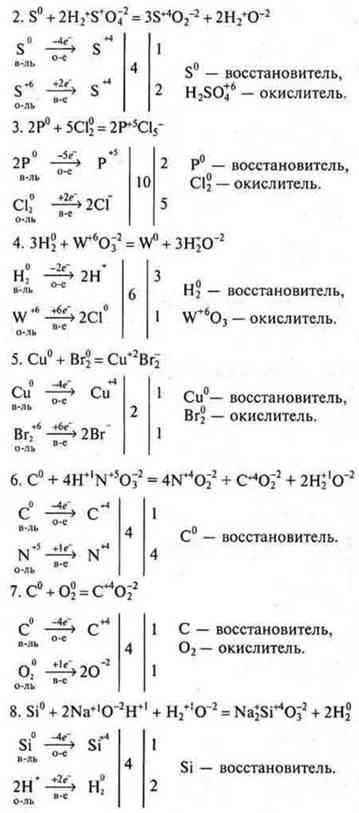
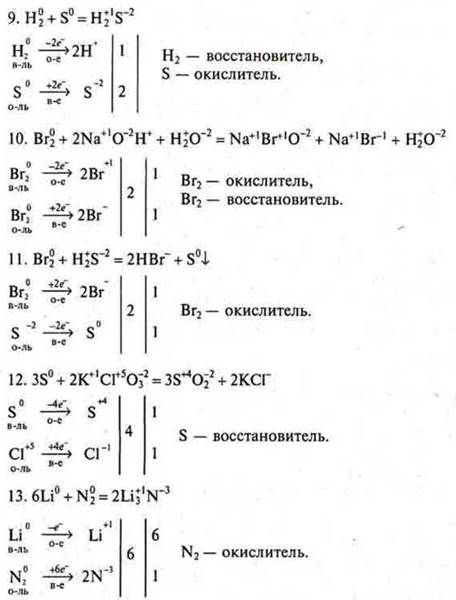
a) the first student: # 5, equations # 2, 9;
second student: # 5. equations # 5, 10;
third student: # 5, equations # 7, 6.
III. Front work
b) after the students ' answers at the blackboard, check from the spot the completion of all remaining questions of task # 5.№ 1, 3, 4, 8, 11, 12, 13.
C) the decision of the task № 5.
Answers to assignment No. 5, § 19

Task # 12
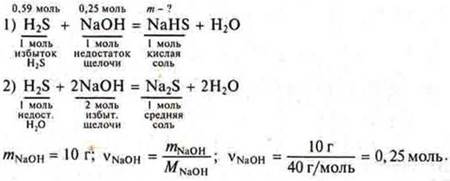
![]()
According to equation (1), it can be seen that H2S is in excess, since 0.25 mol of NaOH requires 0.25 mol of H2S, and 0.59 is given.
Our calculations are based on the equation (1):
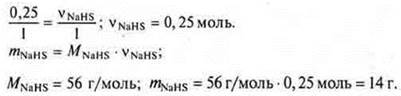
Answer: vvNaHS = 56 g mol; salt is acidic, sodium hydrosulfide.
IV. Learning new material
Plan of presentation
1. Hydrogen compounds of non-metals. Acid-base and reducing properties of hydrogen compounds of non-metals.
2. Oxides formed by non-metals. The acidic nature of oxides of non-metals and change them depending on the position of the element, a nonmetal, forming it into PSHA of D. I. Mendeleev. Oxidative properties of oxides.
3. Hydroxides formed by oxides of non-metals. Change in the character of hydroxides depending on the position of the Mendeleev element-a nonmetal that formed the hydroxide-in the PSC. Oxidative properties of hydroxides.
In the textbook on page 236, the table shows the formulas of hydrogen compounds formed by non-metals. Students work with their teachers on this table, answering questions.
Question. In what S. O. are the nonmetals in the compounds of hydrogen? There are more gases in the aggregate state?
Answer: Since the EO of non-metals is greater than the EO of hydrogen, they act as oxidizers in these compounds, showing the maximum S. O.: C-4, N-3, P-3, etc. Most of these compounds are gases, volatile, easily mobile, quickly vaporized.
Question. Are there any hydrogen compounds известныеthat you know of? Do you know how to get them?
Answer: There are many hydrogen compounds that were studied earlier: CH4— methane, NH3— ammonia, h2O — water, H2S — hydrogen sulfide, HCl — hydrogen chloride. They can be obtained directly by the interaction of a nonmetal with hydrogen:

Question. What kind of chemical bond is present in hydrogen compounds? What type of crystal lattice is characteristic of them? How does the connection's polarity change for a period and for a group, the main subgroup?
Answer: In hydrogen compounds there is a covalent polar bond, they have a molecular crystal lattice.
The polarity of the connection depends on the difference and EO — the larger it is, the more polar the connection is.
Question. How does the connection polarity change in periods? In groups, major subgroups?
Answer: Since the EO increases by the end of the period, therefore, the bonding polarity increases in hydrogen compounds by the end of the period. By the end of the main software group, the EO decreases, and the connection polarity will decrease.
Example: period III hydrogen compounds are arranged in order of increasing polar coupling:
![]()
Strengthening the connection polarity
Hydrogen compounds of group VII, the main subgroup in order of increasing the polarity of the bond:
![]()
Strengthening the connection polarity
Question. Does the polarity of the bond affect the solubility of the hydrogen compound in water? Why?
Answer: it does, because water molecules are strongly polar, and the more polar the molecule, the stronger the intermolecular mutual attraction occurs with the formation of hydrogen bonds.
Question. What compounds are formed when hydrogen compounds of the III period are dissolved in water? Which character — acidic or basic-corresponds to them? And how does it change by the end of the period?
Answer: ![]()
When dissolved in water, there is no manifestation of acid-base character in SiH4, it immediately burns in oxygen; PH3-gives a very weak basic reaction; H2S-gives a weak acidic reaction; HCl — a strong acid reaction, since from SiH4 toHCl the radius of the nonmetal ion decreases, and the charge increases, the polarity of the bond in the molecules increases, all this causes an increase in acidic character.
By the end of the period, the acidic nature of the hydrogen compound increases.
Question. How does the acid-base character of nonmetal hydrogen compounds change in groups and subgroups?
Answer: The acid-base properties of hydrogen compounds of non-metals of the same subgroup differ. The strength of the H—e bond decreases as the bond length increases.
Example: hydrogen compounds of group VII of the main subgroup HF, NSL, HBr, HI; NSL, NVH, HI — these are strong acids that dissociate completely. The strength of the acid increases from HF to HI. HF is the weakest acid. One of the reasons is the smallest radius at F, another reason is the occurrence of intermolecular hydrogen bonds ...H-F...H-F..., which means that the hydrogen atom is connected not only to the f atom of its molecule, but also to the neighboring one.
Conclusion: In periods and groups, the main subgroups, with an increase in the charge of the nuclei of nonmetal elements, acidic and basic properties weaken.
It should be noted that hydrogen compounds in the OVR always exhibit reducing properties, since the element-nonmetal-in the minimum S. O.
Example:

The oxides of non-metals element, non-metal has the positive S. O. is One and the same element, having a few positive S. O., may form several oxides.
Example:

The higher the COC of a nonmetal element in an oxide, the stronger the acidic character of the oxide.
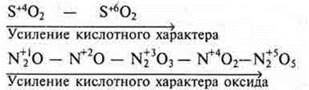
Nonmetal oxides are compounds with a polar covalent bond. Types of crystal lattices-molecular: CO2, SO3; atomic — SiO2.
By the end of the period, the acidic character of the oxide increases.
Example:
![]()
The acidic character of the oxide increases
In groups, the main subgroups, the acidic character of oxides weakens.
Example:
![]()
The acidic character of the oxide is weakened
![]()
The radius of the nonmetal ion increases,
S. O. does not change
Conclusion: All salt-forming oxides formed by non-metals have acidic properties. The strength of the acidic character depends on the S. O. of the nonmetal element and its atomic radius.
Oxides of non-metals correspond to hydroxides — oxygen-containing acids. The change in the strength of an acid depends on the PH and radius of the atom of an element-a nonmetal. We considered the change in the character of oxides formed by the same element.
Example:
![]() -
nitrogen oxides (III, V)
-
nitrogen oxides (III, V)
![]()
The acidic character increases
These oxides correspond to acid hydroxides:
![]()
The acid strength varies in the same way as the oxides, increases S. O. and the radius of the atom nonmetal.
Example: chlorine oxides (I, III, V, VII).
![]() — the
oxides of chlorine (I—VII)
— the
oxides of chlorine (I—VII)
![]()
Strengthening the acidic character of hydroxides-acids
In periods with an increase in the charge of the nucleus of the atom of an element-nonmetal, the acidic properties of hydroxides formed by oxides of nonmetals are observed:
![]()
The acidic character of the hydroxide increases
![]()
C. O. increases; the radius of the nonmetal ion decreases
In groups, the main subgroups, the acidic character of hydroxides formed by oxides of non-metals weakens.
![]()
Weakens the acid character of the hydroxyl,
![]()
The radius of a nonmetal ion increases, but does Not change
All oxides and hydroxides formed by elements in the maximum C. O. are always oxidants.
Example:
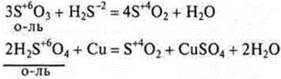
If in the oxide and hydroxide element-nonmetal shows intermediate S. O., then these compounds, depending on the conditions, can be both oxidizers and reducing agents.
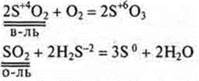
V. Homework assignment
§ 19. To perform tasks on options on separate pieces of the assessment.
I. № 8 (2), № 9 (1), task 14.
II. № 8 (4), № 4 (2), task 15.
VI. Assignment No. 11 § 19.
CO2and SiO22
Similarities: acid oxides, salt-forming, covalent polar bond, SP-hybridization, linear shape of molecules.
Difference: CO2is a gas, andSiO2is a solid. The type of crystal lattice: CO2— molecular,SiO2— atomic.
EOWITH= 2.5 EOO= 3,5SiO2-the connection is more polar
EOSi= 1.8
CO 2 shows a more acidic character2than2Si2, since the radius of the C atom С is less than the radius of the Si atom.
![]() —
carbonic acid,
—
carbonic acid,
![]() correspondsto
H23 Sio3-silicon solid acid,
correspondsto
H23 Sio3-silicon solid acid,
H2Sio33-insoluble,
N2CO3is a weak carbonic acid, soluble.
However, carbonic acid is stronger than silicon. It reacts, like all acids, with metals, metal oxides, and soluble bases.
Similarity: these are dibasic oxygen-containing acids, the S. o. of the element is a nonmetal of the same +4, very weak electrolytes.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.