
Organic and inorganic acids-SUBSTANCES AND their PROPERTIES - LESSON PLANS for CHEMISTRY 11 class - lesson plans-lesson plans-author's lessons-plan-lesson summary - chemistry
The purpose of the lesson: to generalize, consolidate knowledge about the classification, nomenclature, physical and chemical properties of acids: organic and inorganic acids; to teach to explain the common chemical properties of inorganic and organic acids and correctly make reaction equations in molecular and ionic form.
Basic concepts: oxygen-containing and oxygen-free acids, basicity of acids, volatile and non-volatile acid, strong and weak acid, stable and unstable acid, electrochemical series of metal stresses.
Equipment: reagent kit: Mg, Cu, H3S-COOH, C2H5OH,
Cao, ZnO, H2SO4(K), Ag2O(NH3· H2O),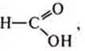 test
tubes, alcohol lamp, test tube holder.
test
tubes, alcohol lamp, test tube holder.
Lesson progress
I. Organizational moment
Setting goals and tasks for the lesson.
II. Checking that homework is completed correctly.
The first student: on the blackboard performs task 2 (Li2O, P2O5, VEO).
Second student: solving problems 1, 2.
III. Front work
Task. Make formulas for oxides of elements # 38, # 56, # 30. Name them, classify them, and create a single equation that confirms their chemical properties.
Answer: Li2O is a basic oxide, VEO — amphoteric oxide, P2O5-acid oxide.
Li2O — the main oxide, methyl oxide, salt-forming, formed by a very active metal, interacts with H2O, acids, acid oxides, amphoteric oxides.
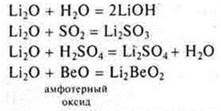
P2O5-phosphorus oxide (V), salt-forming, acidic, formed by non-metal, can interact with water, alkalis, basic and amphoteric oxides.
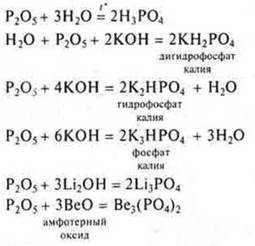
VEO is an amphoteric salt-forming oxide formed by a transition element. As the basic oxide and reacts with acidic oxides and acids.
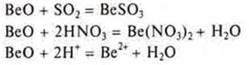
How acid oxide reacts with alkalis and basic oxides.
![]()
By propertiesVEO possible answer: with Li oxides2O I R2About5(in order to save time).
IV. The solution of problems
Task 1
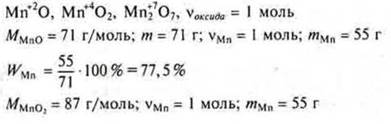
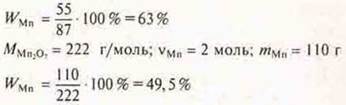
Answer: the largest WMn in oxide MnOh.
3account 2

Answer: 4.9 liters ofCO2 is required.
Formulas of oxides of elements № 38, 56, 30.
No. 38-Sr-strontium. S. O. +2.
SrO-strontium oxide, the main salt-forming oxide солеобразуюшийformed by an alkaline earth element.
![]()
No. 56-Cs-caesium. S. O. +1
Cs2O-caesium oxide, the main oxide, salt-forming, formed by an alkaline metal.
![]()
No. 30-Zn-zinc S. O. +2
ZnO-zinc oxide amphoteric oxide, salt-forming, formed by a transition element, as the main oxide with acid.
![]()
Like oxygen oxide with alkalis
![]()
V. Learning new material
Plan of presentation
1. Definition, classification, nomenclature, chemical bond, type of crystal lattice.
2. Protolytic and electron theories of acid-base properties of compounds.
3. General chemical properties of inorganic and organic acids in the light of the theory of electrolytic dissociation.
a) action on indicators
b) interaction with metals, metal oxides, soluble and insoluble bases, salts.
Task: write out acids From the above formulas of compounds, give them names according to the classification on page 179, scheme 7 of the textbook.
KHSO4, СO2, H2SO4, Cu(OH)2, Н3РO4, HCl, ВаНРO4, H2S, HNO2, Na2SO3, H2SO3, CH3COONa, CH3COOH, HCOOH, C17H35COOK, C17H35COOH.
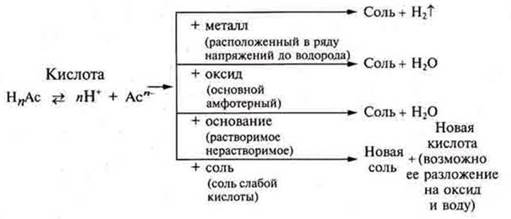
Acids are complex substances that consist of hydrogen atoms that can be replaced by metal and acid residue atoms. General formula: HnAs.
Oxygen-free acids:
HCl-hydrochloric acid,
H2S is hydrosulfuric acid.
Oxygen-containing acids:
H2SO4-sulfuric acid;
N3RHO4-orthophosphoric (phosphoric) acid;
H2SO3— sulfurous acid;
HNO2-nitric acid;
CH3COOH-acetic acid, ethanoic acid;
The HCOOH — methanoic acid (formic acid):
C17H35COOH-stearic acid.
However, there is a more complete classification of acids. In the textbook, it is offered on page 246, table 17. According to this classification, we will describe acids:
H2SO4-sulfuric acid, oxygen-containing, dibasic, soluble, non-volatile, strong, stable.
HCl-hydrochloric acid, oxygen-free, monobasic, soluble, strong, volatile, stable.
H2S — hydrogen sulfide, oxygen-free, dibasic, soluble, weak, volatile, stable.
H2SO3-sulfuric acid, oxygen-containing, dibasic, soluble, unstable, non-volatile, weak.
CH3COOH — ethanoic (acetic) acid, oxygen-containing, monobasic, soluble, volatile, weak, stable.
HNO2-nitric acid, oxygen-containing, monobasic, soluble, weak, non-volatile, stable.
UNLC — methane (formic) acid, oxygen-containing, monobasic, soluble, volatile, weak, stable.
C17H35COOH-stearic acid, oxygen-containing, insoluble, non-volatile, weak, stable.
Next, the teacher offers students the definition of acids in the light of the theory of electrolytic dissociation.
Acids are electrolytes in aqueous solutions in which the cation present in the cation of hydrogen:
![]()
However , in 1923, the protolytic theory of Bernsted-Lauri was proposed, which expanded the understanding of acids and bases. This theory explained the behavior of substances in aqueous and non-aqueous solutions.
According to this theory, acids are molecules or ions that are donors (givers) of hydrogen cations in a given reaction. +:
![]()
The acid is a donor of the hydrogen cation. The hydrogen cation is called a proton, so the theory is called protolytic. According to the electronic theory of acids and bases of the American chemist G. N. Lewis, one of the connecting atoms gives up its free electron pair for the formation of a chemical bond, and the other atom provides its free electron cell (orbital). The first atom is an electron donor, the second atom is an acceptor (receiving) - a chemical bond is formed by the donor-acceptor mechanism.
In accordance with this, G. N. Lewis formulated a new concept of acids and bases. Acids are those reagents that are electron acceptors. Bases are those reagents that act as electron pair donors.
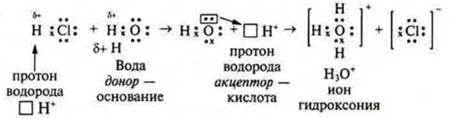
H3O+ is the hydroxonium ion. It is present in solutions of all acids, but it can also act as an acid — it gives off a proton and turns into a water molecule. In the reaction equations, for simplicity of writing, we will use the designation of the hydroxonium — H+ ion.
The General chemical properties of organic and inorganic acids: sour taste, color change of indicators, interaction with metals, basic and amphoteric oxides, soluble and insoluble bases, salts, are caused by hydrogen cations in solutions.
Dissociation in aqueous solutions
Strong acids, α → 1:
![]()
Weak acids, α → 0:
![]() -
step dissociation
-
step dissociation
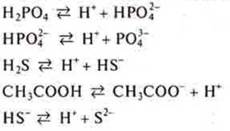
Changing the color of indicators:
a) methyl orange-pink color;
b) phenolphthalein — no change;
C) blue litmus — red color;
d) universal indicator - from yellow to pink color.
Further, the teacher experimentally proves the properties of acids, students independently write down on the blackboard and in notebooks the equations of reactions of interaction with metals located in the electrochemical series of stresses of metals to hydrogen.
Experiment:
![]() -
inorganic acid
-
inorganic acid
![]()
Experiment:
![]() -
organic acid
-
organic acid
![]()
Interaction with basic and amphoteric oxides
Experiment:
![]() -
inorganic acid
-
inorganic acid
![]()
Experiment:
![]() —
organic acid
—
organic acid
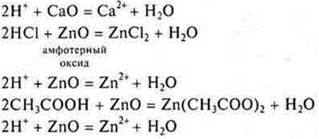
Equations of reactions with amphoteric oxide students independently write down on the blackboard and in notebooks.
Interaction with soluble and insoluble bases
Polybasic acids can form acidic salts and medium salts. These are neutralization reactions.
Soluble base:
![]() -
inorganic acid
-
inorganic acid
![]() -
inorganic acid
-
inorganic acid
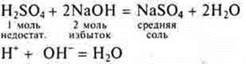
![]() -
organic acid
-
organic acid
Insoluble base
Experiment:
![]() -
inorganic acid
-
inorganic acid
![]()
Experiment:
![]() —
organic acid
—
organic acid
![]()
Interaction with salts
It should be remembered that a strong acid can displace a weak acid even from an insoluble salt.
Experiment
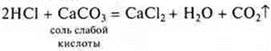 —
inorganic acid
—
inorganic acid
![]() — gas
and water are formed
— gas
and water are formed
Experiment:
![]()
![]() -
organic acid Generalization of the General chemical properties of inorganic and
organic acids should be written in the form of a diagram.
-
organic acid Generalization of the General chemical properties of inorganic and
organic acids should be written in the form of a diagram.
VI. Homework assignment
§ 20 to p. 248. Nos. 1, 2, properties of H2SO4.
VII. Pinning
Task: Which of the listed substances will interact with H3PO4: K,SiO2, Zn(OH)2, Na2SО4, MgCО3, PbO, Cu? Draw up the corresponding reaction equations in molecular and ionic form.
N3RHO4-phosphoric acid,
N3RO4-acid of medium strength, in the solution there are ions N2RO4-; NRO42 -and RO43-. Фосфат-ионовThere are fewer phosphate ions, but their binding occurs in the precipitate, and it is possible to write an equation with dissociation to the phosphate ion.
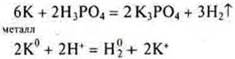
![]() — not
interact
— not
interact
![]() — not
interact
— not
interact
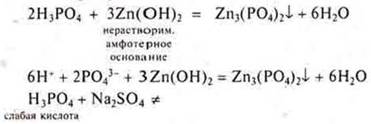
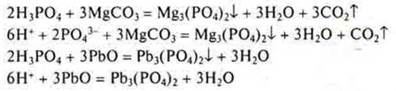
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.