
Organic and inorganic bases-SUBSTANCES AND their PROPERTIES - LESSON PLANS for CHEMISTRY 11 class - lesson plans-lesson plans-author's lessons-plan-lesson summary - chemistry
Lesson objectives: to consolidate knowledge about the classification, nomenclature, physical and chemical properties, structure reason: organic and inorganic; chemical properties reason: to teach correctly write the equations of reactions, confirming the chemical properties of bases; to generalize and to consolidate the theory of structure of organic compounds on the mutual influence of atoms and groups of atoms.
Basic concepts: base, donor, acceptor, protolytic theory and electronic base theory, mutual influence of atoms and groups of atoms in organic compounds.
Equipment: reagent kit, NH3· H2O, test tubes, test tube holder, alcohol lamp, SASO3, gas outlet tube.
Lesson progress
I. Organizational moment
Setting goals and tasks for the lesson.
II. Independent work
|
Option I |
Option II |
|
1. Acidic properties are most pronounced in the substance: |
|
|
|
|
|
2. The ion equation of the reaction |
|
|
|
|
|
corresponds to the interaction: |
|
|
a) potassium hydroxide and nitric acid b) barium hydroxide and sulfuric acid C) lithium hydroxide and barium chloride d) ammonia and bromine of hydrogen acid |
a) sodium carbonate and acetic acid b) calcium carbonate and nitric acid C) calcium bicarbonate and hydrochloric acid d) barium carbonate and formic acid |
|
3. Formulas of products of interaction of concentrated sulfuric acid with |
|
|
silver: and) H2and Ag2SO4 b) SO2, H2O and Ag2SO4 C) H2S, H2O4and Ag2SO4 d) the reaction is not |
magnesium: a) the reaction does not go b) H2S, H2O, MgSO4 C) SO2, H2O, MgSO4 g) H2, MgSO4 |
|
4. Set a match: left side of the molecular equation: |
|
|
|
|
|
right side of the ion equation: |
|
|
|
|
|
Task with a free response 5. Draw up reaction equations that confirm the properties |
|
|
of nitric acid |
and sulfuric acid |
|
6. Print the formula of the acid |
|
|
|
|
Answers to self-study questions
Option I
1. G.
2. a.
3. b.
4. 1-g; 2-a; 3-b; 4-V.
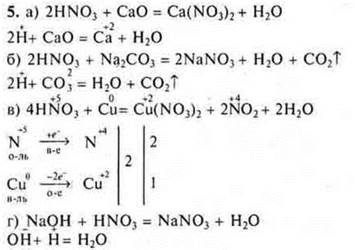
6. Task
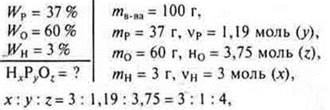
N3RHO4-phosphoric acid.
Option II
1. G.
2. a.
3. b.
4. 1-V; 2-a; 3-g; 4-V.
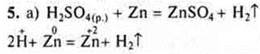
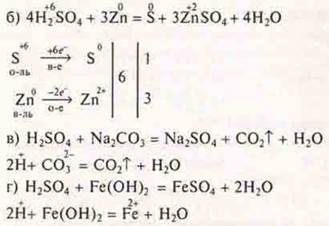
6. Task
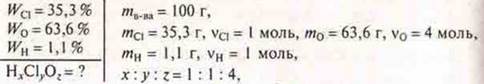
HC-4is a perchloric acid.
III. Checking that homework has been completed correctly
At the time of completing the test, the teacher checks the completion of homework in the workbooks of some students.
Answers to homework questions
§ 20, № 3
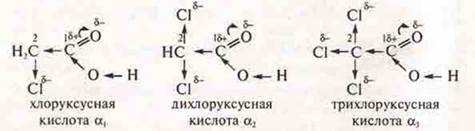
α3> α2> α1; in the molecule trichloroacetic acid δ+ carbon No. 1 increased by mixing the electron density at the π-connection in the direction of oxygen and mixing of the electron density to the more electronegative chlorine atoms in trichloroacetic — to three chlorine atoms, monochloroacetic in — one chlorine atom, which causes the greatest amount of displacement δ - from the oxygen atom in the group —HE, providing the mobility of the protons in the group —HE and easy dissociation of acids, particularly trichloroacetic acid.
§ 20 № 5
![]()
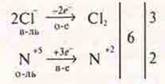
§ 20 № 6
![]()
HF-molecule is stronger than HCl, because the atom F has a radius of the atom less than the atom Withl, and EOF> EOCl. The greater the difference in EO, the more polar the bond, the stronger the molecule.
The hcl acid is stronger than HF, since the radius Of the CL ion is greater than the radius of the f-non, as well as the core charge of Cl+17, a F+9. The proton in the HCl molecule repels with the greatest force, and dissociation is easy.
αHCl> α>HFhydrogen bonds are formed between highly polar HF molecules and polar water molecules
![]() which
makes HF dissociation more difficult.
which
makes HF dissociation more difficult.
Task 9

IV. Learning new material
Plan of presentation
1. determination of bases, classification, nomenclature, chemical bond, type of crystal lattice.
2. Protolytic and electronic nature of the acid-base properties of bases.
3. Chemical properties of inorganic and organic bases in the light of the theory of electrolytic dissociation:
a) action on indicators;
b) interaction with acids, salts, oxides.
The teacher asks students to find entries related to the classification of substances in their workbooks. Find the definition of bases and their classification.
General
formula of bases: ![]()
Bases — complex substances, consisting of metal atoms associated with one or more hydroxocuprate.
Example:
Fe(OH)3— iron(III) hydroxide; NaOH-sodium hydroxide.
Bases are soluble — alkalis, poorly soluble and insoluble. However, in table 19 p. 252 of the textbook, a more thorough classification of bases is given, which we need to know.
Example:
Fe(OH)3-iron (III) hydroxide, oxygen-containing , triacid, insoluble, weak α → 0, non-volatile, unstable.
NaOH-sodium hydroxide, oxygen-containing, monoacid, soluble, strong, α → 1, non-volatile, stable.
CH3-NH2-organic base methylamine, oxygen-free, monoacid, soluble, weak, α → 0, volatile, stable.
From
the point of view of the theory of electrolytic dissociation, bases are
electrolytes, in whose aqueous solutions the hydroxogroup anion is present as
an anion: ![]()
In the light of the Brensted-Lowry protolytic theory, bases are molecules or ions that serve as proton acceptors. According to the electronic theory of G. N. Lewis, bases are those reagents that act as donors of the electron pair.
Example:
NH3— ammonia, the nitrogen atom has an unsheltered electron pair, therefore: NH3acts as a donor, i.e. a base.
At
organic bases-amines, in the amino group-![]() at the
nitrogen atom there is an undivided pair of electrons, therefore, amines —
organic bases.
at the
nitrogen atom there is an undivided pair of electrons, therefore, amines —
organic bases.
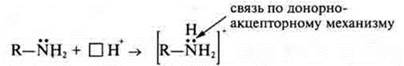
If we consider inorganic bases, then the chemical bond in the base molecule between the metal cation and the hydroxogroup is ionic, and in the hydroxogroup — covalent-polar. A no-type crystal lattice can be both ionic and molecular.
Chemical properties of inorganic bases
Soluble inorganic bases dissociate:
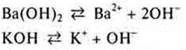
Anion gidroksipropil causes a change in color of indicators:
phenolphthalein-crimson color;
blue litmus — does not change;
methyl orange-yellow;
universal indicator-up to blue color.
Interaction of soluble and insoluble bases with acids
The experiment is conducted by a teacher, students independently write down the reaction equations on the blackboard and in notebooks.
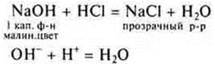
Experiment:
![]()
If there is a lack of HCl, the main salt is formed.
![]()
Interaction of soluble bases with acid oxides
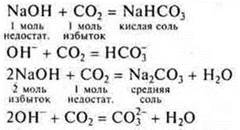
Experiment:
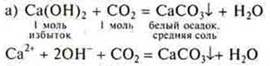
Skip FROM2until the sediment disappears
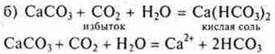
![]()
When conducting this experiment, it is necessary to once again explain to students the concepts of "excess" and "lack" in the formation of different salts of polybasic acids. You need to know this before solving calculation problems.
The interaction of soluble salts with bases
Experiment:
![]()
Interaction of soluble bases with amphoteric oxides and amphoteric bases
Different products are formed: everything depends on the conditions.
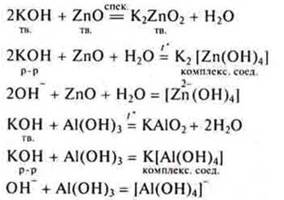
Interaction of soluble and insoluble bases with organic substances
![]()
Decomposition of insoluble bases by heating
Experiment:
![]()
As a generalization, you can make a diagram:
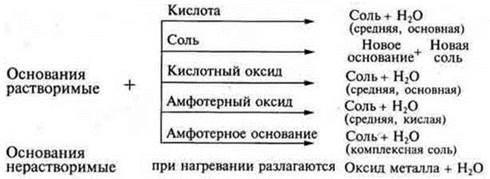
Under certain conditions, the insoluble base reacts with acid oxides, always reacts with acids.
Organic bases — amines, heterocyclic compounds. The composition of amines includes an amino group that provides the main properties of organic compounds. In properties they are similar to ammonia, but amines as the base limit of the number of stronger.
Let's compare the basic character of ammonia and methylamine.
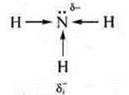 In
ammonia, the δ-atom of nitrogen increases due to the shift of
the electron density from three hydrogen atoms.
In
ammonia, the δ-atom of nitrogen increases due to the shift of
the electron density from three hydrogen atoms.
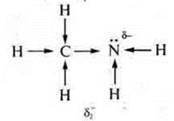 In
methylamine, the δ-nitrogen atom increases not only due to the
electron density of hydrogen atoms, but also the radical —Ch3.
δ2- > δ1-
In
methylamine, the δ-nitrogen atom increases not only due to the
electron density of hydrogen atoms, but also the radical —Ch3.
δ2- > δ1-
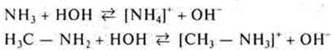
Aromatic amines have weaker basic properties. The aromatic ring, the π-electron cloud (6e-) comes into conjugation with an unshielded pair of electrons of the nitrogen atom in the group —NH2, thereby reducing the δ- nitrogen atom, the protonation weakens. In the aromatic ring, density is redistributed. Carbon atoms have the highest electron density at positions 2, 4, and 6.
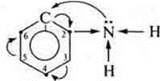
In these positions, the substitution reaction will easily take place. This is due to the mutual influence of atoms and groups of atoms in organic compounds (the position of the theory of chemical structure of A. M. Butlerov).
 —
theheterocyclic compound pyridine is the base.
—
theheterocyclic compound pyridine is the base.
Many heterocyclic compounds of more complex composition are the basis of nitrogenous bases: thymine, adenine, guanine, cytosine, which are the basis of DNA, RNA.
Many medicinal products contain derivatives of aromatic amines.
V. Homework assignment
1) § 21, № 2, 3, 4, 5.
2) Draw up equations of reactions confirming the main properties:
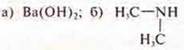
Do your homework on a separate sheet for evaluation.
VI. Pinning
§ 21, № 1.
1) phenol-acid
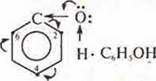 Mobility
of a hydrogen atom in a group-OH is explained as follows:
Mobility
of a hydrogen atom in a group-OH is explained as follows:
a) an unshared pair of electrons of an oxygen atom of group-OH comes into contact with the 6E-π cloud of the aromatic ring;
b) an oxygen atom, group-ON, losing its electronic density, will mix in its direction the bond with hydrogen, providing it with mobility:
C) in the aromatic ring, the electron density is redistributed. The largest amount is obtained by C atoms С at positions 2, 4, and 6. this provides an easy substitution of hydrogen atoms.
The amino group-NH2has exactly the same effect on the
aromatic ring in the phenyl-amine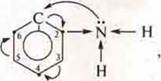 ,
so that the substitution of hydrogen in the carbon positions 2, 4, 6 is easy,
and the amine itself becomes a weak organic base.
,
so that the substitution of hydrogen in the carbon positions 2, 4, 6 is easy,
and the amine itself becomes a weak organic base.
The total impact is AboutH in phenol and-NH2in amine on the aromatic ring is a change in the electron density in the ring, and the acquisition of its greatest in the positions of carbon atoms 2, 4, 6.
- HE and-NH2are orientants of the first kind.
According to the theory of the structure of organic compounds by a.m. Butlerov, the properties of substances depend not only on which atoms and how many of them are part of a molecule, but also on how they are connected and mutually affect each other.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.