
Amphoteric organic and inorganic compounds-SUBSTANCES AND their PROPERTIES - LESSON PLANS for CHEMISTRY class 11 - lesson plans-lesson plans-author's lessons-lesson plan-lesson summary - chemistry
Lesson objectives: to consolidate knowledge of the concept of "aftermost", to teach to explain the amphoteric properties of inorganic and organic compounds and write the equations of chemical reactions; to consolidate the knowledge about complex compounds; to draw conclusions about the relativity of the terms "acid" and "base"; to improve practical skills of performing chemical experiments, observing the rules of health and safety.
Basic concepts: amphotericity, amphoteric compounds, acid, base, complex compound, relativity of the concepts "acid" and "base", polymer, polypeptide, polycondensation reaction.
Equipment: a set of reagents, test tubes, NH, H2O, AlCl3— on the teacher's table and for laboratory experience on the students ' table.
Lesson progress
I. Organizational moment
Setting goals and tasks for the lesson.
II. Checking that homework has been completed correctly
Answers to homework tasks
§ 21 № 2
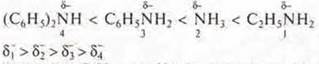
ethylamine C2H5→ NH2; δN-increases due to the radical-C2H5, its electron density and the electron density of two h atoms, providing a strong protonation, the main character.
NH3— ammonia; δN-- amplification only by the electron density of three h atoms, the main character, i.e. protonation is weaker.
![]() -
phenylamine, an aromatic amine,δn-decreases.
t. K. an unsheltered pair of nitrogen electrons comes into contact with the 6E-π
cloud of the aromatic ring, and protonation weakens. the basic character is
reduced.
-
phenylamine, an aromatic amine,δn-decreases.
t. K. an unsheltered pair of nitrogen electrons comes into contact with the 6E-π
cloud of the aromatic ring, and protonation weakens. the basic character is
reduced.
![]() —
diphenylamine, an aromatic amine:δn-decreases even
more strongly compared to the previous one, because the unsheltered pair of
electrons of the nitrogen atom comes into contact with the 6E-π
clouds of two aromatic tracks, protonation is very weak, the basic properties
are very small.
—
diphenylamine, an aromatic amine:δn-decreases even
more strongly compared to the previous one, because the unsheltered pair of
electrons of the nitrogen atom comes into contact with the 6E-π
clouds of two aromatic tracks, protonation is very weak, the basic properties
are very small.
§ 21 № 3
The main properties within the same period, the hydrogen compounds are reduced to the end of the period because the nuclear charge of atom increases gradually, the ion radius of the element decreases, and S. O. element increases, the protonation weakens, enhanced by the acidic nature of the recoil proton.
Example: hydrogen compounds of period II elements:
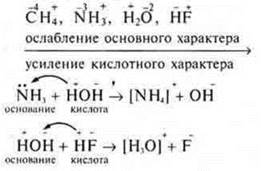
§ 21 № 4
The main properties of volatile hydrogen compounds of one group by the end of the group the main subgroup weaken. S. O. element does not change, the rapidly growing charge of the nucleus of an atom element ion and increases the radius of the atom, ion, protonation weakens. the recoil of the proton increases, i.e. the acidic character.
VI group, main subgroup
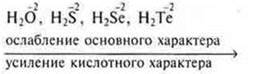
§ 21 № 5
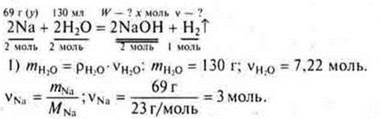
![]() a mol
Na is required, and given 3 mol ⇒× Na
is a disadvantage. The calculation is based on the"lack".
a mol
Na is required, and given 3 mol ⇒× Na
is a disadvantage. The calculation is based on the"lack".
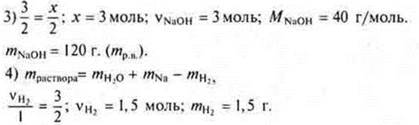
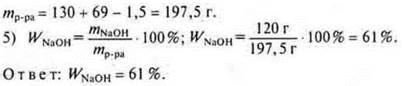
III. Learning new material
Plan of presentation:
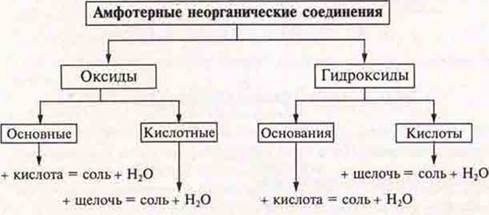
1. Amphotericity, amphoteric inorganic compounds.
2. Laboratory experience. Obtain an amphoteric aluminum hydroxyl and prove its amphoteric properties.
3. Compilation of generalizing schemes of amphoteric compounds: oxides and hydroxides.
4. Amphoteric organic compounds.
5. Relativity of the concepts "acid" and "base".
Amphotericity (variability) — the manifestation of a compound of acidic or basic properties, depending on the conditions.
Amphoteric compounds-oxides and hydroxides, as a rule, are formed by transition elements.
Students should clearly remember the transition elements that are most often present in connections.
Elements with S. O. +2 — Be, Zn; S. o. +3 — Al, Withr, Fe; S. O. +4 — Sn, Pb.
They form oxides and hydroxides with acid-base properties.
|
Element |
Oxide |
Hydroxide-base |
Hydroxide-acid |
|
Be |
|
Ine(HE)2 |
N2VEO2 |
|
Zn |
|
Zn(OH)2 |
H2ZnO2 |
|
Al |
|
AlOH)3 |
H3AlO3-aluminum acid (orthoform) Nalo2-metaaluminic acid (metaform) |
|
Withr |
|
Withr(HE)3 |
N3CGO3-chromic acid (orthoform) Nsgo12-methachromic acid (metaform) |
|
Pb |
|
Pb(ON)4; PbAbout(HE)2; (PBO · mon2O) |
H4PBO4(orthoform) H2PbO3(meta form) |
Conditions of flow reactions:
- substances are taken in solid form and sintered. As a rule, are formed of solid compounds metaformin or orthoformic;
- substances are taken in solutions, complex compounds are formed.
The teacher reminds students that they learned about these compounds while studying the topic "Classification of salts". Notebooks contain entries about their structure, classification, and nomenclature.
Students together with the teacher begin to perform laboratory experiments to obtain amphoteric aluminum hydroxide and experimental proof of its amphotericity.
Experiment:
Get aluminum hydroxide by having Alcl3and NH3· H2O:
![]()
Observation: a translucent gelatinous precipitate falls out — this is aluminum hydroxide.
Students share the received Al(HE)3 for two test tubes. In one, they leave most of Al(HE)3; Al(HE)3-very weak acid, insoluble.
Experiment:
To prove itsacidicproperties, we calculate the reaction Al(OH)3with an alkali solution.
Students add NaOH to Al(HE)3, where the smaller part is.
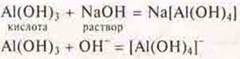
Observation: sediment Al(HE)3disappears, a complex compound is formed.
Giving a characteristic:
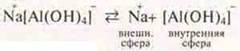
![]() —
structure of the inner sphere:
—
structure of the inner sphere:
![]() —
complexing agent;
—
complexing agent;
4 (HE)- - ligands.
The coordination number (C. h.) of the complexing agent is 4.
This is an anionic complex, since the inner sphere is negatively charged. Name: sodium Tetra-hydroxo-aluminate.
Experiment:
To prove the basic properties of Al(HE)3perform the reaction, add to Al(HE)3stronger acid:
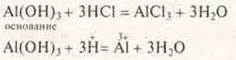
Observation: sediment Al(HE)3disappears. Thus, we experimentally confirmed the manifestation of amphotericity — the acid-base properties of aluminum hydroxyl.
The teacher focuses the attention of students on the similarity of recording reactions and experiments with compounds of chromium, gallium, etc. of all elements with S. O. +3.
Then the teacher asks students to draw up equations of reactions that confirm the amphotericity of aluminum oxide — to be a basic and acidic oxide.
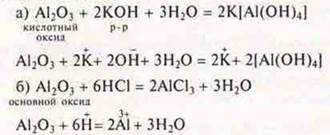
In the case of sintering, using a substance in solid form, the teacher writes down the reaction equations:
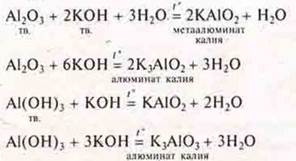
The scheme is compiled as a generalization:
In organic chemistry, typical amphoteric compounds are amino acids. General formula for α-amino acids:
![]()
—: NH2-amino group provides amino acids with basic properties, i.e. amino acids interact with acids.
Example:
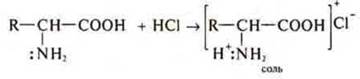
- COOH-carboxyl group provides amino acids with acidic properties. They interact as acids with alkalis, salts, metals, and metal oxides.
Example:
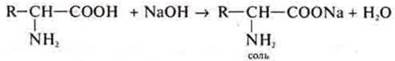
If the amino acids are in solution, they dissociate to form a bipolar ion:
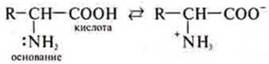
Most importantly, due to the amphotericity of amino acids at the cellular level, they interact with each other, and polypeptides — proteins are formed:
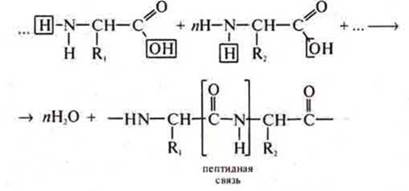
The General formula of the polypeptide:
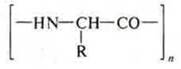
In industry as a result of polycondensation reaction of amino acids with terminal locations of groups-NH2 and-CUN synthesize polymers-synthetic fibers. Example: kapron fiber.
At the end of studying the question of amphoteric compounds, the teacher explains to students the relativity of the concepts "acid" and "base" on the example of the interaction of water with ammonia.
In this reaction, water is the keyto the slot, since it gives away a proton (protolytic theory) , and ammonia is the base, since it takes a proton (protolytic theory), and provides an unassigned pair of electrons-a donor, also a base (electronic theory):
![]()
When water reacts with acids, in the case of acid dissociation, for the same reasons, water is the base:
![]()
This question is difficult to understand in ordinary classes. It is desirable to offer this information to students with increased motivation to learn.
If you arrange the compounds in order of increasing their acidic properties, you will get a series of:
NH3< N2H4< H2O < HCN < H2S < CH3COOH < N3RHO4< HF < HNO3< HCl < H2SO4< HBr < HClO4.
It turns out that every substance in this series, with the exception of the extreme ones, shows amphotericity, depending on what substance it interacts with. Regarding the division of acids into strong and weak, and bases into strong and weak: it all depends on what solvents it interacts with.
Example: when a weak CH3COOH acid is dissolved in water, it acts as an acid:
![]()
And if you dissolve it in sulfuric acid, then it acts as a base:
![]()
CH3COOH — weak acid, when dissolved in H2O dissociates little, but when dissolved in NH3 (liquid), which as a base is stronger than water, CH3COOH dissociates much more strongly, turns out to be as strong as sulfuric acid in vol.
Alcohols in aqueous solutions do not exhibit acidic properties, but in NH3liquid they exhibit acidic properties:
![]()
Thus, one of the important conclusions of the proton-electron theory of acids and bases is the relativity of the concepts "acid" and"base".
III. Homework assignment
§ 22 № 2, 3, 4; prepare for practical work № 4 on the topic "Hydroxides".
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.