
Practical work # 5 on the topic "the Genetic relationship between classes of inorganic and organic compounds" - SUBSTANCES AND their PROPERTIES-LESSON DEVELOPMENTS in CHEMISTRY 11 class - lesson developments-lesson developments-author's lessons-plan-lesson summary - chemistry
The purpose of the lesson: to improve practical skills and skills of performing chemical experiments, observing the rules of OT and TB; to teach experimentally carry out the transformation of substances according to the scheme, make observations, write down the equations of chemical reactions, draw conclusions.
Equipment: reagent kits, alcohol lamp, matches, test tube holder; C2H5OH, CAC2, Al, phenol, acetic acid, solution I2.
Lesson progress
I. Organizational moment
Setting goals and tasks for the lesson. Instructing students to perform practical work on tasks in compliance with the rules of OTT and TB. All reactions are recorded in molecular and ionic form. The work is done in pairs.
II. Performance of tasks
|
Option I |
Option II |
|
|
|
1. Experimentally perform the transformation according to the scheme, write down all the observations, make up the equations of reactions, draw the appropriate conclusions
2. A thought experiment.
Make a diagram of transformations, write down the equations of reactions according to this scheme:
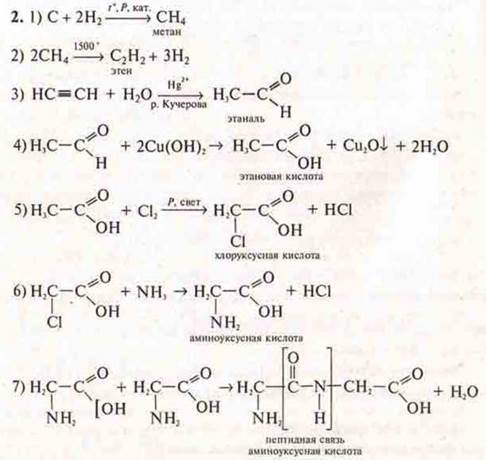
![]() C
→ dipeptide glycine-glycine
C
→ dipeptide glycine-glycine
III. Summarizing the lesson results
IV. Homework assignment
1) Repeat § 17-23. Prepare for the General lesson.
2) Draw up the transformation schemes and write the reaction equations according to the compiled scheme:
a) ethane → ... → polystyrene;
b) CAS2→ ... → 2,4,6-trinitrotoluene.
Answers to practical work tasks
Option I
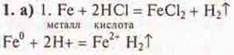
The release of hydrogen gas is observed.
![]()
A dark green precipitate of iron (II) hydroxide falls out.
![]()
On air Fe(OH)2 oxidizes to Fe(OH)3-iron (III) hydroxide of brown color.
![]()
The sediment disappears.
![]()
The sediment disappears.
Iron (III) sulfate salt is formed, the yellow color of the solution.
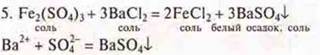
There is a white precipitate — barium sulfate.
![]()
Iron precipitates and accumulates on the zinc granule.
Conclusion: a genetic series of iron was proposed, where a genetic relationship of classes was observed: metal (simple substance), salt, insoluble bases, salt, salt, simple substance — metal.
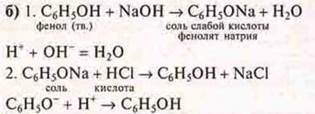
The appearance of pinkish clusters of phenol in the form of balls is observed.
![]()
Conclusion: a genetic series of compounds with an aromatic ring was proposed.
![]()
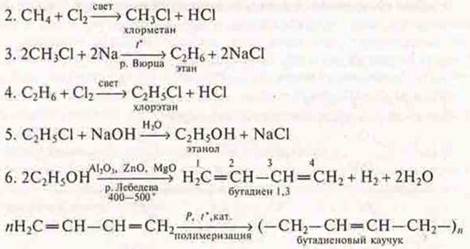
Option II
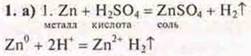
The release of hydrogen gas is observed.
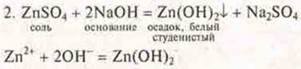
There is a precipitation of white, gelatinous zinc hydroxide.
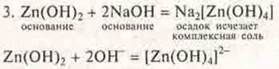
The precipitate disappears, the formed complex salt is Tetra-hidroxizina sodium.
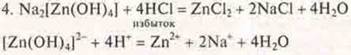
Under the action of an excess of acid, medium salts and water are formed.
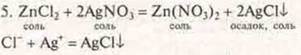
There is a white flake — like precipitate-silver (I) chloride.
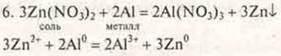
Zinc is deposited on the aluminum granule.
Conclusion: a genetic series of zinc was proposed, where a genetic relationship of classes was observed: metal-simple substance, salt, base, complex salt, salt, salt, simple substance-metal.
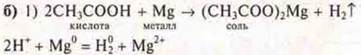
The release of hydrogen gas is observed.
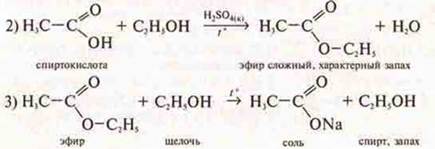
There is an alkaline hydrolysis.
Conclusion: the scheme of transformations of organic substances was performed: acid, salt, acid, ester complex, salt.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.