
Lesson of generalizing repetition on the topic "Substances and their properties" - SUBSTANCES AND their PROPERTIES - LESSON plans FOR CHEMISTRY 11 class-lesson plans-lesson plans-author's lessons-plan-lesson summary - chemistry
Lesson objectives: to summarize and consolidate key issues, themes, concepts, teach reasonably explain the classification, chemical properties of the studied classes of substances, properly prepare the reactions according to the schemes of transformations, to apply knowledge to solving computational problems.
Equipment: tests-tasks for each table.
Recommendations to the teacher: in Gesta, tasks of various levels are given. The teacher offers students tasks of their choice, but for each studied topic question.
Lesson progress
I. Organizational moment
Setting goals and tasks for the lesson. Organization of students to work in order to prepare for test work No. 4 (pair work).
II. Execution of the test and discussion of correct answers
Test question
1. Classification of substances:
a) distribute substances by classes of inorganic compounds:
SG(ON)3, Nclo4, NVG, NaH2PO4, SO2, (CuOH)2CO3, Cu2O, K[Al(OH)4]. Call them.
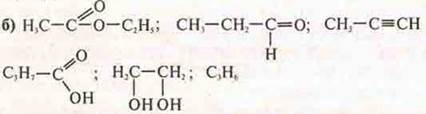
Specify classes of organic compounds and name them.
2. Metals:
a) Draw up electronic and graphical formulas of atoms: Zn, Na, MP; define S. o. Draw up formulas of higher oxide and hydroxide, indicate their nature.
b) Using the electrochemical series of metal stresses, give at least two examples of reactions that characterize the chemical properties of metals and their compounds:
![]()
3. Nonmetals:
a) Make electronic formulas of bromine, nitrogen, and sulfur atoms. Write down the formulas of the hydrogen compound, the higher oxide, and the hydroxide.
b) Determine how the acidic character of oxides in the series changes
SO3- P2O5- SiO2-H2O
Give a reasonable answer.
C) Show how bond strength (breaking energy) changes in a number of substances
H2O - H2S – H2Se - H2Te
Please explain the answer.
4. Acid organic and inorganic:
a) with which of the proposed substances will sulfuric acid interact (DIB.)? Make reaction equations in molecular and ionic form: si, FeO, SO2, si(OH)2, BCL2, Na2S.
b) Arrange Hydrobromic, iodo-Hydrobromic, chloro-Hydrobromic, and hydrofluoric acids in order of increasing their acidic properties. Explain the answer.
C) Consider the mutual influence of atoms in molecules of methane and ethanoic acids. Which acid is stronger? Please explain the answer.
5. Base organic and inorganic:
a) How do the properties of volatile hydrogen compounds of elements of group V of the main subgroup of the PSC change with increasing ordinal number? Give a reasonable answer.
b) the Interaction of which substances corresponds to the short ionic equations:
![]()
C) How do the main properties of volatile hydrogen compounds of elements of the II period of the PSCE change with increasing ordinal number? Give a reasonable answer.
6. Amphoteric compounds:
a) will it change the color of the blue litmus in aminoacetic acid? Justify the answer.
b) draw up formulas for oxides and hydroxides of the following cations and indicate their nature: SG2+, SG3+, SG6+.
C) when carefully adding potassium hydroxide solution to the zinc sulfate solution, a precipitate is first formed, which dissolves when an excess of alkali is added. Explain the answer. Draw up equations of chemical reactions.
7. Genetic connection of inorganic compounds:
a) From substances whose formulas are S, si, SO2, H2SO4, BaSO4, SO3, Na2SO3, make a genetic series (complete), write down the reaction equations.
b) from substances whose formulas are si, si, NO2, si(OH)2, CCL2, NCL, make a genetic series (complete), write down the reaction equations.
C) Write the reaction equations according to the transformation schemes:
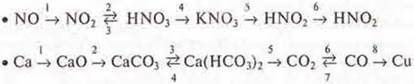
8. Genetic connection of organic compounds.
a) From compounds of the formula which
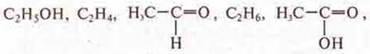
make a genetic series and equations of chemical reactions of all transformations.
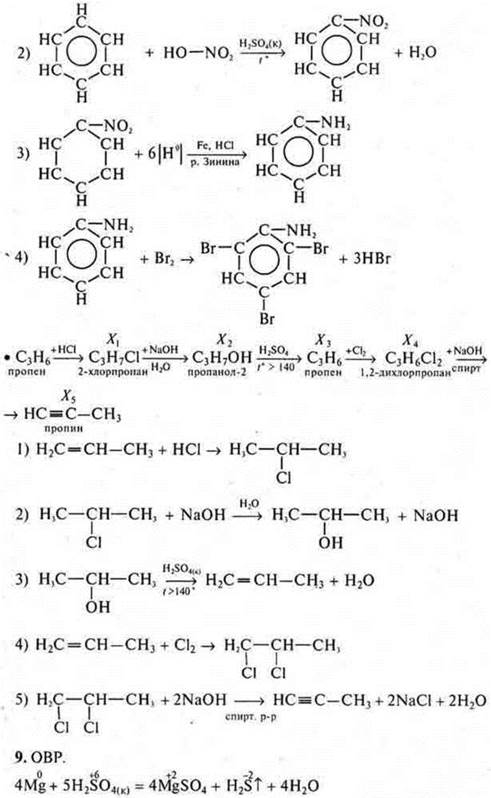
b) Draw up equations of reactions according to the transformation schemes:
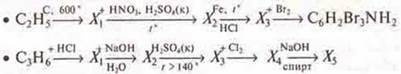
To give names to all the substances.
9. Oxidation-reduction reactions:
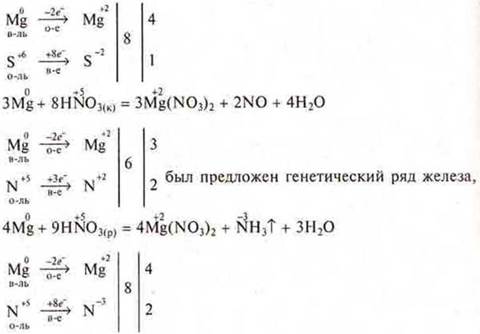
a) Make an IAB between Mg and H2SO4(K); HNO3(K), HNO3(p.).
b) Give definitions:
* the reducing agent is...
• the oxidizing agent is...
C) Determine the S. O. of elements in compounds and answer the question: as an oxidizer or reducing agent or both, depending on the conditions, they act: K2SG2O7; K2MPO4; NH3.
d) create an IAB:
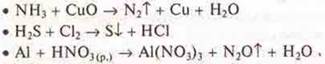
10. Calculation tasks:
a) problems on the extraction of formulas of the compound.
• The combustion of organic matter weighing 2.52 g produced 7.92 g of carbon monoxide (IV) and 3.24 g of water. The vapor density of this substance in the air is 2.9. Output the molecular formula of this organic substance.
* Print the molecular formula of a fluoro-derived marginal hydrocarbon with a mass fraction of fluorine 73 %, carbon 23 % and hydrogen 4%. The relative molecular weight of this compound is 52.
b) Problems in the chemical equation with the concepts of "excess" and "lack".
* Calculate the mass of the reaction product, if you take 37.2 g of aniline and 146 g of hydrochloric acid 20% for it.
C) Problems on the chemical equation with the mass fraction of the product yield.
• By reacting ethanol weighing 27.6 g with copper (II) oxide, 25 g of acetic aldehyde was obtained. Calculate the mass fraction (in%) of the yield of aldehyde to the theoretically possible.
Answers to the test questions
1. a) salt-Forming oxides:
Si2O-copper oxide (I) - basic oxide;
SO2-sulfur oxide (IV) - acid oxide.
Acids:
HClO4-perchloric acid: oxygen-containing, monobasic, soluble, non-volatile, strong, stable;
NVG-Hydrobromic acid: oxygen-free, monobasic, soluble, strong, volatile, stable.
Footings:
SG(ON)3Chromium (III) 3-hydroxide: oxygen-containing, triacid, insoluble, amphoteric, non-volatile, weak, unstable.
Salts:
NaH2PO4-sodium dihydrogen phosphate, acidic salt, soluble;
(Zion)2CO3-hydroxycarbonate of copper (II), basic salt, insoluble;
![]() —
complex compound, potassium Tetra-hydroxo-aluminate, anionic complex.
—
complex compound, potassium Tetra-hydroxo-aluminate, anionic complex.
b) C4H10-the ultimate hydrocarbon, alkane. CnH2n+2; butane.
C3H6— CnH2n-either an alkene: propene, unsaturated, or cycloalkane-cyclopropane.
H3S-S≡SN-alkine, unsaturated, propine.
![]() —
oxygen-containing, extreme aldehyde, propanal.
—
oxygen-containing, extreme aldehyde, propanal.
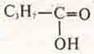 -
oxygen-containing limiting acid-butanoic acid.
-
oxygen-containing limiting acid-butanoic acid.
![]() —
oxygen-containing, limit, ester, ethyl ether of ethanoic acid.
—
oxygen-containing, limit, ester, ethyl ether of ethanoic acid.
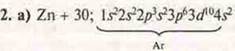 -
d-element, transition metal.
-
d-element, transition metal.
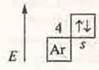
S. O.+ 2.
ZnO-zinc oxide-amphoteric oxide. Zn(OH)2-amphoteric hydroxide. The zinc hydroxide.
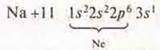 —
s-element is an alkaline metal.
—
s-element is an alkaline metal.
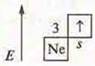
S. O.+ 1
Na2O is sodium oxide, the main oxide. NaOH-sodium hydroxide, soluble base, alkali.
![]() -
d-metal element with transition properties.
-
d-metal element with transition properties.
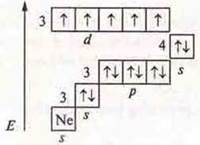
S. O. +2, +4, +7.
S. O. + 7, maximum. MP2O7— oxide of manganese (VI), the acidic oxide. Nmpo4-manganese acid, hydroxide.
b)
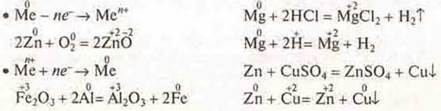
3. The non-metals.
![]() -
p-element, non-metal
-
p-element, non-metal
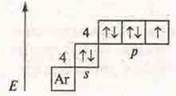
![]() —
hydrobromide is a hydrogen compound.
—
hydrobromide is a hydrogen compound.
![]() -
bromine oxide.
-
bromine oxide.
HBrO4-acid;- bromine acid; hydroxide-acid.
N + 7 1s22s22p3p-element, non-metal.
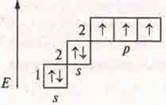
![]() —
hydrogen compound, ammonia.
—
hydrogen compound, ammonia.
N2O5oxide of nitrogen (V).
HNO3— nitric acid; hydroxide-acid.
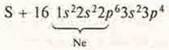 R-element,
non-metal.
R-element,
non-metal.
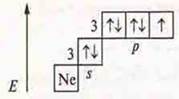
![]() —
hydrogen compound, hydrogen sulfide.
—
hydrogen compound, hydrogen sulfide.
![]() —
sulfur oxide (VI).
—
sulfur oxide (VI).
H2SO4-sulfuric acid; hydroxide-acid.
b) acidic character in the row from left to right decreases:
![]() T. S.
C. E. of the element is reduced and the ion radius of the element increases.
T. S.
C. E. of the element is reduced and the ion radius of the element increases.
C) in the row H2O — H2S — H2Se-H2The strength of the bond decreases from left to right, as the polarity of the bond decreases (the difference in EO), the charge of the nonmetal ion core and its radius increase.
4. H2SO4(p )interacts with:
FeO-basic oxide; si(OH)2— insoluble base; Vasl2-salt, will precipitate.
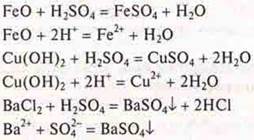
5. HF < HCl < HBr < HI:
HI is the strongest acid, by the end of this series, the radius of the nonmetal ion and the charge of its core increases, which causes the strongest repulsion of the proton-cation of hydrogen, dissociation is easy.
6. a) NH3> PH3> AsH3> SbH3> BiH3.
As the ordinal number increases, the charge of the nonmetal ion's core increases, and the radius of the nonmetal ion increases, which causes the main properties to weaken — to be a donor of a pair of electrons, and the acidic properties increase,
b) NH3> N>2ABOUT > HF.
Basic properties of hydrogen compounds by the end of the period weakened, because by the end of the period an increasing nuclear charge of the ion-nonmetal increases his S. O., decreasing the radius of the ion-nonmetal, enhanced acidic properties to be donor — proton- cation of hydrogen.
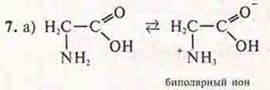
The solution is neutral, pH = 7. The blue litmus becomes purple.
b![]() — -
chromium (II) oxide, the main oxide;
— -
chromium (II) oxide, the main oxide;
SG(ON)2Chromium (II) 2-hydroxide, insoluble base;
![]() —
chromium (III) oxide, amphoteric oxide;
—
chromium (III) oxide, amphoteric oxide;
SG(ON)3Chromium (III) 3-hydroxide, amphoteric base;
![]() —
chromium (VI) oxide, acid oxide;
—
chromium (VI) oxide, acid oxide;
H2CGO4-chromic acid.
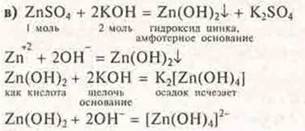
In excess of alkali, zinc hydroxide shows acidic properties in this case.
The precipitate disappears, because it formed a soluble complex compound of zinc Tetra-hidroxizina potassium.
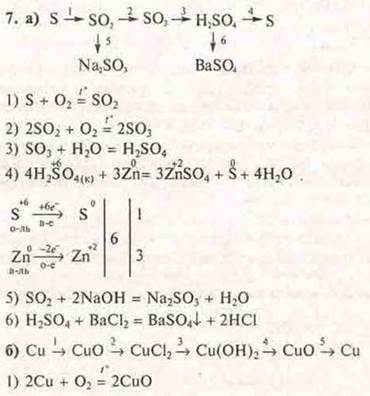
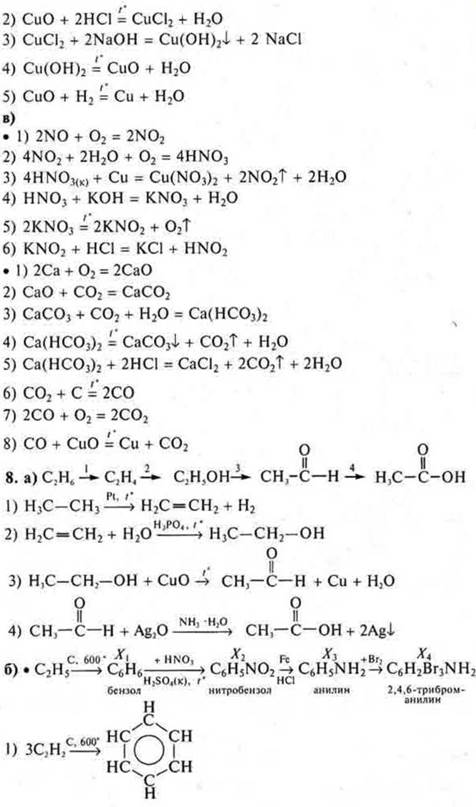
b) reducing Agent-a particle (a molecule, ion, atom) that increases the level of PSA in the OVR (gives up electrons — it is oxidized). An oxidizer is a particle (a molecule, ion, atom) that lowers the level of PSR (takes electrons — it is restored).
C
—![]() - in
the maximum S. O. it is an oxidizer;
- in
the maximum S. O. it is an oxidizer;
![]() — in
the intermediate S. O., depending on the conditions, it can be both an oxidizer
and a reducing agent;
— in
the intermediate S. O., depending on the conditions, it can be both an oxidizer
and a reducing agent;
![]() — in
the minimal S. O. only the reducing agent.
— in
the minimal S. O. only the reducing agent.
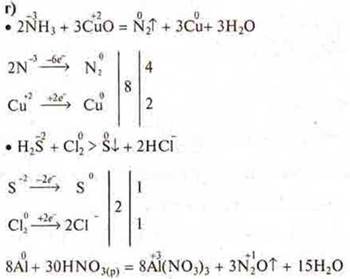
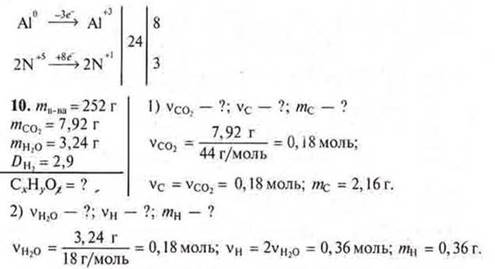
3) mO= 2,52 - 2,16 - 0,36 = 0 — there is no oxygen in the compound.
4) x : y = 0.18 : 0.36 = 1 : 2.
CH2is the simplest formula, mCH2= 14 g/mol.

3)![]() y
= 0.8 mol of aniline required, and given 0.4 ⇒×
aniline in deficit. The calculation is based on a lack.
y
= 0.8 mol of aniline required, and given 0.4 ⇒×
aniline in deficit. The calculation is based on a lack.
4) ![]()
Answer: the mass of the reaction product is 51.8 g.
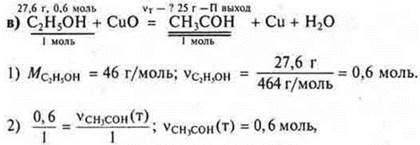
m
theoretical output ![]()
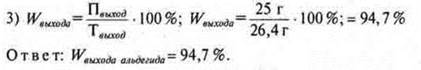
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.