
Introduction
What kind of system are you: open or closed? As it turns out, this is a physics question, not a philosophical one. You, like all living things, are an open system, meaning that you exchange both matter and energy with your environment. For instance, you take in chemical energy in the form of food, and do work on your surroundings in the form of moving, talking, walking, and breathing.
All of the exchanges of energy that take place inside of you (such as your many metabolic reactions), and between you and your surroundings, can be described by the same laws of physics as energy exchanges between hot and cold objects, or gas molecules, or anything else you might find in a physics textbook. Here, we’ll look at two physical laws – the First and Second Laws of Thermodynamics – and see how they apply to biological systems like you.
Systems and surroundings
Thermodynamics in biology refers to the study of energy transfers that occur in molecules or collections of molecules. When we are discussing thermodynamics, the particular item or collection of items that we’re interested in (which could be something as small as a cell, or as large as an ecosystem) is called the system, while everything that's not included in the system we’ve defined is called the surroundings.
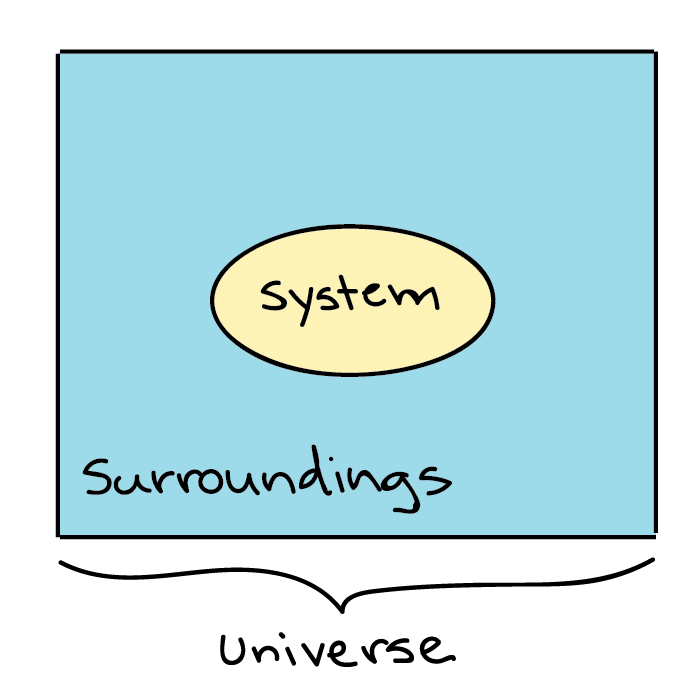
Generalized depiction of the system (a circle), the surroundings (a square surrounding the circle), and the universe (system + surroundings).
For instance, if you were heating a pot of water on the stove, the system might include the stove, pot, and water, while the surroundings would be everything else: the rest of the kitchen, house, neighborhood, country, planet, galaxy, and universe. The decision of what to define as the system is arbitrary (up to the observer), and depending on what you wanted to study, you could equally well make just the water, or the entire house, part of the system. The system and the surroundings together make up the universe.
There are three types of systems in thermodynamics: open, closed, and isolated.
· An open system can exchange both energy and matter with its surroundings. The stovetop example would be an open system, because heat and water vapor can be lost to the air.
· A closed system, on the other hand, can exchange only energy with its surroundings, not matter. If we put a very tightly fitting lid on the pot from the previous example, it would approximate a closed system.
· An isolated system is one that cannot exchange either matter or energy with its surroundings. A perfect isolated system is hard to come by, but an insulated drink cooler with a lid is conceptually similar to a true isolated system. The items inside can exchange energy with each other, which is why the drinks get cold and the ice melts a little, but they exchange very little energy (heat) with the outside environment.
[Why is a cooler sometimes called a "closed" system?]
You, like other organisms, are an open system. Whether you think about it or not, you are constantly exchanging energy and matter with your surroundings. For instance, suppose that you eat a carrot, or lift a bag of laundry onto a table, or simply breathe out and release carbon dioxide into the atmosphere. In each case, you are exchanging energy and matter with your environment.
Exchanges of energy that take place in living creatures must follow the laws of physics. In this regard, they are no different from energy transfers in, say, an electrical circuit. Let's take a closer look at how the laws of thermodynamics(physical rules of energy transfer) apply to living beings like yourself.
The First Law of Thermodynamics
The first law of thermodynamics thinks big: it deals with the total amount of energy in the universe, and in particular, it states that this total amount does not change. Put another way, the First Law of Thermodynamics states that energy cannot be created or destroyed. It can only change form or be transferred from one object to another.
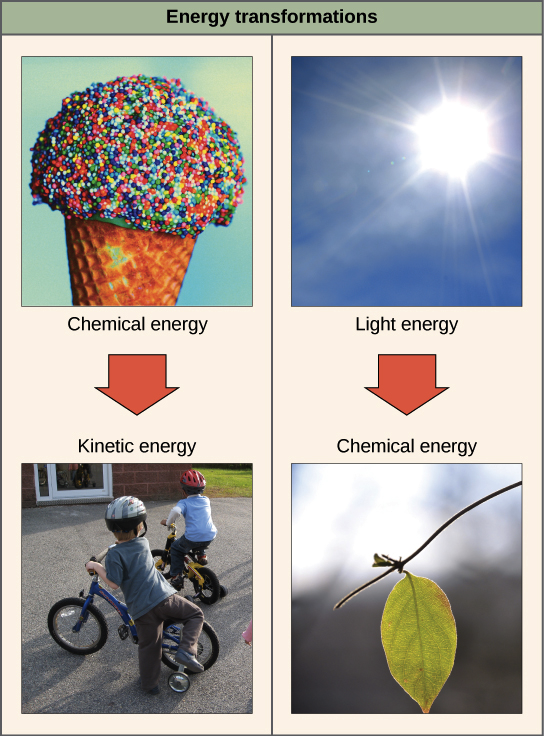
Image of ice cream cone (chemical energy) being converted to motion of kids riding bikes (kinetic energy).
Image of sun (light energy) being converted to sugars in a leaf (chemical energy).
Image credit: OpenStax Biology. Credit “Ice cream," modification of work by D. Sharon Pruitt; credit "Kids on bikes," modification of work by Michelle Riggen-Ransom, and credit “Leaf”: modification of work by Cory Zanker.
This law may seem kind of abstract, but if we start to look at examples, we’ll find that transfers and transformations of energy take place around us all the time. For example:
· Light bulbs transform electrical energy into light energy (radiant energy).
· One pool ball hits another, transferring kinetic energy and making the second ball move.
· Plants convert the energy of sunlight (radiant energy) into chemical energy stored in organic molecules.
· You are transforming chemical energy from your last snack into kinetic energy as you walk, breathe, and move your finger to scroll up and down this page.
Importantly, none of these transfers is completely efficient. Instead, in each scenario, some of the starting energy is released as thermal energy. When it's moving from one object to another, thermal energy is called by the more familiar name of heat. It's obvious that glowing light bulbs generate heat in addition to light, but moving pool balls do too (thanks to friction), as do the inefficient chemical energy transfers of plant and animal metabolism. To see why this heat generation is important, stay tuned for the Second Law of Thermodynamics.
The Second Law of Thermodynamics
At first glance, the first law of thermodynamics may seem like great news. If energy is never created or destroyed, that means that energy can just be recycled over and over again, right?
Well…yes and no. Energy cannot be created or destroyed, but it can change from more-useful forms into less-useful forms. As it turns out, in every real-world energy transfer or transformation, some amount of energy is converted to a form that’s unusable (unavailable to do work). In most cases, this unusable energy takes the form of heat.
Although heat can in fact do work under the right circumstances, it can never be turned into other (work-performing) types of energy with 100% efficiency. So, every time an energy transfer happens, some amount of useful energy will move from the useful to the useless category.
Скачано с www.znanio.ru
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.