
Worksheet
Reversible and irreversible processes. Entropy. The second law of thermodynamics.
Lesson objectives:
- knows the wording of the second law of thermodynamics;
- can explain the meaning of the second law of thermodynamics;
- applies the second law of thermodynamics in the analysis of thermodynamic processes.
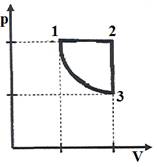
(W) Task 1. The graph shows the process of transition of an ideal gas of constant mass from the state of 1-2-3-1. In the first state, the gas is under pressure of 0.8 ∙ 105 Pa, occupying a volume of 5 m3. Being in state 3, it occupies a volume two times larger than in the first one and has a temperature of 800 K.
(I) Determine the amount of substance of the gas.
(II) Determine the gas temperature and volume in the second state.
(III) Determine the volume of gas in state 3.
(IV) What work did the gas do during the isobaric expansion?
Question: How has the internal energy of a gas changed for a cycle? Can we say that then, during the cycle, the gas received an amount of heat equal to 4 * 105 J?
(G) Task 2. Solve the task in pairs.
Discuss the solution in pairs, then hold a general discussion with the teacher.
Suppose a cup of coffee with a temperature 800С is cooling in a room with a temperature 200С, loses Q =1000 J heat.
А) Determine the entropy change ΔS cup of coffee.
В) Determine the entropy change ΔS air in the room.
С) Determine the total entropy of the system.
(G) Task 3. Solve tasks in pairs. After joint discussion and solving the assignment, the students compare their answers with the answers of the next couple in pairs.
1. The irreversible process is
a) a process that can occur both in the forward and in the opposite direction
b) a process that does not reverse spontaneously
c) a process that takes place without the influence of external bodies
2. Choose a formulation that is not relevant to the second law of thermodynamics.
a) The amount of heat supplied to the system goes to change its internal energy and to perform the system work.
b) Heat itself cannot move from a colder body to a warmer one.
c) In nature, a process is impossible, the only result of which would be mechanical work done by cooling the heat reservoir
d) It is impossible to design a machine that will only transfer heat from a colder body to a warmer one.
3. The second law of thermodynamics reflects ...
a) orientation of thermodynamic processes in closed systems
b) energy conservation in thermodynamic processes
c) the transformation of energy from one type to another in thermodynamics
d) the ability of internal energy to spontaneously transform into work
(I) Task 4. Individual problem solving.
(G) Task 5. Solve tasks in pairs. After a joint discussion and solving the assignment, the students compare their answers with the answers of the next couple, prove the correctness and falsehood of the statements.
Determine which statements are true and which are false.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.