
Theoretical material for the lesson, definitions for concepts
Ideal gas equation
It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law, Charles's law and Avogadro's Law.So far, we have seen how p, V and T are related. It is possible to write a single equation relating these quantities which takes into account the amount of gas being considered.
If we consider n moles of an ideal gas, we can write the equation in the following form:
pV = nRT
This equation is called the ideal gas equation or the equation of state for an ideal gas.
Kinetic Theory of ideal gases (Assumption for Ideal gases)
To describe an ideal gas, a set of assumptions are made.
1) Gases consist of large numbers of tiny particles that are far apart relative to their size. This implies that the gas molecules have negligible volume compared to the volume of container in which they are placed.
2) Collisions between gas particles and between particles and container walls are elastic collisions (there is no net loss of total kinetic energy).
3) Gas particles are in continuous, rapid and random motion. They therefore possess kinetic energy, which is energy of motion.
4) There are no forces of interaction between gas particles. Thus they can move independent of each other. They only interact with each other through elastic collisions.
5) The average kinetic energy of a gas particle depends only on the temperature of the gas.
Avogadro's Hypothesis
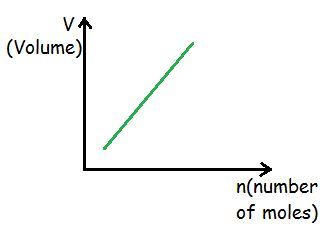
As the number of gas particles increases, the frequency of collisions with the walls of the container must increase. This, in turn, leads to an increase in the pressure of the gas. Flexible containers, such as a balloon, will expand until the pressure of the gas inside the balloon once again balances the pressure of the gas outside. Thus, the volume of the gas is proportional to the number of gas particles.
If pressure and temperature of an ideal
gas is kept constant then volume of container is directly proportional to the
amount of gas (number of moles of gas) in the container.
Dalton's Law of Partial Pressures
Imagine what would happen, gases at different pressure but same temperature are added to a container. The total pressure would increase because there would be more collisions with the walls of the container. There is so much empty space in the container that each type of gas molecules hits the walls of the container as often in the mixture as it did when there was only one kind of gas. The total pressure will increase as more number of gas molecules hits the container walls but the pressure due to individual gas molecules remains same. The total number of collisions with the wall in this mixture is therefore equal to the sum of the collisions that would occur when each gas is present by itself. In other words,
The total pressure of a mixture of gases
is equal to the sum of the partial pressures of the individual gases.
Additional guidelines for organizing a lesson
|
(W) Greeting. Teacher greets and registers students. (W) Review of previous lesson. Ask learners. Questions: What do you mean by Avogadro's number? What is its value? How do you calculate the Molar Mass of a Substance? What is the absolute zero temperature? Why is it called that? What is the physical meaning of temperature? Explain the lesson topic, learning and lesson objectives. State the new lesson and tell students the importance of lesson. Task : Working in groups of 2 or 3 students think about and describe the phrase ‘ideal’ in different contexts. How can we combine the meaning of ‘ideal’ with the characteristics of the gases? |
|
(G) Work with resource Bilimland. What is the equation of state for an ideal gas? A gas whose molecules have zero volume and which do not interact with one another, apart from the moments when they collide. What is the equation of state for an ideal gas? Activities 1-2 (Bilimland-3) (G) Propose students to independently derive and explain the equation of state for the ideal gas. Individual students are invited to find brief information about the work of Clapeyron.
R =k
The gas equation of state links the basic quantities that characterise a gas. The equation is often applied in the following form: pV=nRT The Clapeyron equation combines all the gas parameters. (W) Students and teacher will solve one or two examples. Students will individually solve problems that require the application of formula: (pV = NRT); (I) After which teacher gives worksheet task to student to individually complete. Call the weak students on the board to solve the problems. Check the students’ answers and give the feedbacks to them. Individual work with weak students. Give the extra worksheets to the students who solved all the problems. Criteria: To achieve the learning objective, learners must correctly solve at least five out of six problems (83 %) on ideal gas laws. Exercises and Problems: 1. 10 m3 of butane gas at 1.2 atm are to be stored at 6.0 atm, at the same temperature. To what volume must the gas be compressed to give the required storage pressure? 2. A metallic cylinder contains methane at 20 oC at a pressure of 8.0 atmospheres. After some heating, its temperature goes up to 40 oC. What is the new pressure, in atm, inside the container? A. 8.0 B. 16.0 C. 8.5 D. 4.0 E. 7.5 3. What is the volume, in dm3, of 6.0 g of chlorine (Cl2) at 27 oC and 101 kPa? 4. A 5.0 L container holds 0.50 kg of butane gas (C4H10). Assuming ideal gas behavior, calculate the pressure of the gas if the gas is stored at 25 oC. 5. 13.9 grams of a noble gas are placed in a 5.0 L container at a pressure of 58.6 kPa and a temperature of 60.0 °C. Which gas is it? 6. Determine the volume of occupied by 2.34 g of carbon dioxide gas at standard temperature and pressure (STP)? 7. If the atmospheric pressure on Mars is found to be about 0.0079 atm, what is the density of CO2 at a pressure of 0.0079 atm and 227 K on Mars? (Note: Use the values of constants in the formulae and methods described in the accompanied PPT. These are the approximate atmospheric conditions on Mars.) Checklist of Learning Objectives: o recall and understand phases of matter; o recall and understand laws of ideal gas; o recall and solve problems using the equation of state for an ideal gas expressed as рV = nRТ; o describe a simple kinetic model for solids, liquids and gases; |
|
Teacher facilitates discussion with students on lesson: Reflect on lesson objectives—achieved or not; etc. Teacher issues the homework assignment. |
Additional multilevel (on differentiation) tasks
The graph shows how the pressure of an ideal gas varies with its volume when the mass and temperature of the gas are constant.
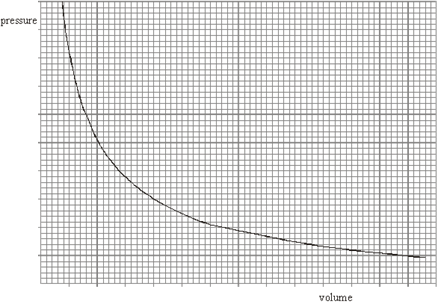
(a) On the same axes, sketch two additional curves A and B, if the following changes are made.
(i) The same mass of gas at a lower constant temperature (label this A).
(ii) A greater mass of gas at the original constant temperature (label this B). (2)
(b) A cylinder of volume 0.20 m3 contains an ideal gas at a pressure of 130 kPa and a temperature of 290 K. Calculate
(i) the amount of gas, in moles, in the cylinder,
______________________________________________________________
______________________________________________________________
______________________________________________________________
(ii) the average kinetic energy of a molecule of gas in the cylinder,
______________________________________________________________
(iii) the average kinetic energy of the molecules in the cylinder.
______________________________________________________________
______________________________________________________________
______________________________________________________________(5)(Total 7)
Mark scheme
(a) (i) curve A below original, curve B above original (1)
(ii) both curves correct shape (1) 2
(b) (i) (use of pV = nRT gives) 130 × 103 × 0.20 = n × 8.31 × 290 (1)
n = 11 (mol) (1) (10.8 mol)
(ii)
(use of Ek = ![]() kT gives)
Ek =
kT gives)
Ek = ![]() ×
1.38 × 10–23 × 290 (1)
×
1.38 × 10–23 × 290 (1)
= 6.0 × 10–21 J (1)
(iii)
(no. of molecules) N = 6.02 × 1023 × 10.8 (= 6.5
× 1024)
total k.e. = 6.5 × 1024 × 6.0 × 10–21 =
3.9 × 104 J (1)
(allow C.E. for value of n and Ek from (i) and
(ii))
(use of n = 11 (mol) gives total k.e. = 3.9 (7) × 104
J) 5
[7]
List of useful links and literature
https://www.khanacademy.org/test-prep/mcat/chemical-processes/thermodynamics-mcat/v/pv-diagrams-part-2-isothermal-isometric-adiabatic-processes
David Sang, Graham Jones, Gurinder Chadha and Richard Woodside. Cambridge International AS and A Level. Coursebook. Physics Second Edition. Chapter 22. Page 351
Mike Crundell, Geoff Goodwin and Chris Mee. Cambridge International AS and A Level. Physics. Second Edition. Topic 10. Page 202
https://brilliant.org/wiki/ideal-gas-law/
https://medium.com/countdown-education/5-things-you-should-know-about-pv-nrt-aka-the-ideal-gas-law-2b11d9bab373
https://physics.gurumuda.net/the-ideal-gas-law.htm
https://opentextbc.ca/introductorychemistry/chapter/the-ideal-gas-law-and-some-applications-2/
Скачано с www.znanio.ru
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.