
THERMODYNAMIC PROCESS EXAMPLE #1
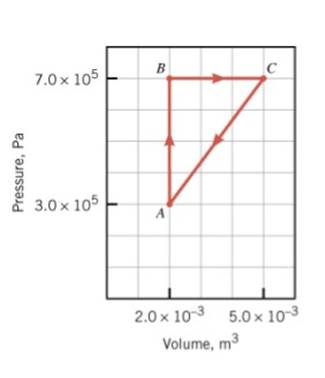
COMPLETE THE TABLE BELOW FOR THE PROCESS ABOVE.
|
|
SPECIAL PROCESS |
Q (J) |
W (J) |
ΔU (J) |
|
A à B |
|
|
|
|
|
B à C |
|
|
|
|
|
C à A |
|
|
|
|
|
TOTAL |
|
|
|
|
THERMODYNAMIC PROCESS EXAMPLE #2
A mole of an ideal gas starts at ½ atmosphere and 1.00 m3 (Point A). The gas pressure increases by 2.5 atm due to an isochoric process (to POINT B). The gas then expands under a linear process to a volume of 3.0 m3 and its original pressure (POINT C). Finally the gas contracts under an isobaric process back to its original volume (BACK TO POINT A).
1 2 3
Volume (m3)
|
Point |
P (kPa) |
V (m3) |
T (K) |
U (kJ) |
|
A |
|
|
|
|
|
B |
|
|
|
|
|
C |
|
|
|
|
|
Step |
U (kJ) |
Q (kJ) |
W (kJ) |
|
A – B |
|
|
|
|
B – C |
|
|
|
|
C - A |
|
|
|
|
Cycle |
|
|
|
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.