
Laboratory work №9. "Measurement of the charge of one valence ion".
The purpose of the work is to determine the Faraday number and the electron charge.
Instruments and accessories: electrolytic bath with a solution of copper sulphate, DC source, stopwatch.
Laws of electrolysis
Let the charge of one ion be Ze, where e is the elementary charge, Z is the valence of the ion, i.e. the number of electrons donated or acquired during dissociation by each atom. The charge given to the electrode is equal to
q = n∙Z∙e, (1)
where n is the number of ions.
On the other hand, the mass M of the substance released on the electrode is equal to
М = n∙m, (2)
where m is the mass of one ion.
From formulas (1) and (2) we find
М= ![]() ∙q.
(3)
∙q.
(3)
It is known that the same number of atoms in one molecule of ν of any substance contains: N = NA,
NА = 6,023· 10![]() mol
mol![]() (Avogadro’s
number).
(Avogadro’s
number).
Then the mass of the ion (we neglect the masses of the two electrons cut off from the atom) will be equal to
m=![]() ,
(4)
,
(4)
where μ is the molar mass
Substitute the expression (4) in (3), we get
М = ![]() (5)
(5)
Magnitude
![]() ,
(6)
,
(6)
the constant for each substance is called the electrochemical equivalent of this substance. Thus, the mass M of the substance released on the electrode is proportional to the charge q transmitted through the electrolyte (the first Faraday law).
The magnitude of the charge q transmitted through the electrolyte is equal to the product of the current strength I by the time t of its passage: q = I · t.
Therefore, the electrochemical equivalent (taking into account (5) and (6)) can be calculated by the formula
К = ![]() .
(7)
.
(7)
From the formula (6) we find
А= ![]() .
(8)
.
(8)
The value![]() is called
the chemical equivalent of a substance..
is called
the chemical equivalent of a substance..
From equation (8) it follows that the chemical equivalent of substances is proportional to its electrochemical equivalent (the second Faraday law):
К = ![]() .
(9)
.
(9)
The constant F is called the Faraday number.
Expression (7) can be rewritten as
М = ![]() q .
(10)
q .
(10)
In order for the substance to stand out on the electrode, numerically equal to A, it is necessary to pass the charge q = F through the electrolyte.
Consequently, the Faraday number F is numerically equal to the magnitude of the charge, when passing through the electrolyte on the electrode, a mass of substance is released that is numerically equal to A.
From expressions (8), (9) it follows that
F = ![]() = NAe. (11)
= NAe. (11)
The charge of a monovalent ion is equal to the charge of an electron in absolute value. Therefore, the electron charge can be calculated by the formula
е = ![]() .
(12)
.
(12)
Working process
1. Dry the copper electrode used as a cathode on a hot plate for 10 minutes and weigh it on a laboratory scale. The value of the measured mass M1 is recorded in Table 1.
2. Place copper electrodes - the cathode and the anode in a glass bath with electrolyte. Connect the cathode to the negative terminal of the DC source, the anode to the positive terminal
3. Turn on the power source, simultaneously start the stopwatch, making sure that the power source is working in the current stabilization mode. Pass current through the electrolyte for 30-40 minutes.
4. Turn off the power source and the stopwatch, dry the cathode on the electric plate for 10 minutes and use its balance to determine its mass M2. The difference M1 – M2 gives the mass increment of the cathode M. The results of the measurements are recorded in the table.
5. Using formulas (7), (11), (12), calculate the values of the electrochemical equivalent, the Faraday number and the electron charge. When calculating, it should be assumed that for copper = 63.510-3 kg / mol and in the compound copper is divalent - Z = 2.
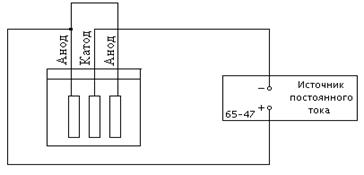
![]() Picture. 1
Picture. 1
The results of the calculations in the table.
|
М1, кg |
М2, кg |
М, кg |
I, А |
t, s |
К, кg/C |
F, C/мol |
е, |
|
|
|
|
|
|
|
|
|
Questions for admission to work
1. What is the electrochemical equivalent? What is the chemical equivalent called? What is their physical meaning?
2. Determine the purpose of the work.
3. Describe the working setup and write down the working formulas.
4. Estimate
the accuracy of this method of measuring the Faraday number and the electron charge.
![]()
Вопросы для защиты работы
1. What conductors are called conductors of the first and second kind?
2. Formulate the laws of electrolysis.
3. What is the relationship between the Faraday number, the chemical and electrochemical equivalent of a substance, and what is the physical meaning of the Faraday number F?
4. Is it possible to determine the electrochemical equivalent if an alternating current is passed through the bath?
5. After the experiment, you found that the polarity of the electrodes was mistakenly changed. Is it possible in this case to determine the electrochemical equivalent?
6. What are your critical comments on this work?
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.