
Mark Scheme
1. B
2. C
3. D
4. C
5. B
6. C
7. Mean kinetic energy of atom µ absolute temperature [1]
![]() µ T or v2
µ
µ T or v2
µ ![]() [1]
[1]
Since the mass m of the atom is constant, we have: v µ ![]() [1]
[1]
The temperature of 0 °C in kelvin is T = 273 K
The absolute temperature increases by a
factor of ![]() (= 36.6) [1]
(= 36.6) [1]
Hence the speed will increase by a factor of ![]() = 6.05 [1]
= 6.05 [1]
The speed of the atoms at 10 000 K =
1.3 × 6.05 » 7.9 km s–1
[1]
8. aThe particles have a range of speeds and travel in different directions. [1]
b i) Mean kinetic energy = ![]() [1]
[1]
= 1.118 ´ 10–19 J » 1.1 ´ 10–19 J[1]
b ii)![]() [1]
[1]
![]() [1]
[1]
speed = 1.147 ´ 104 m s–1 » 11 km s–1[1]
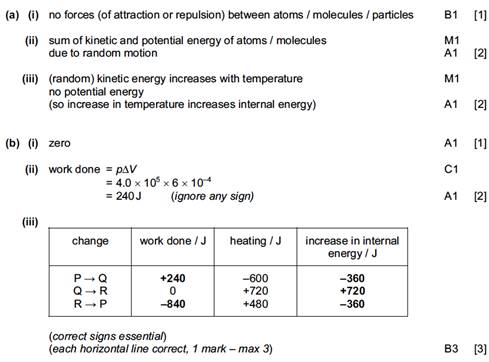
9. (a) Realization that since pV = constant, the temperature must be the same i.e. 400 K / full calculation using gas law to get 400 K; 1
(b) (i) work done is area under curve;
and this is ![]() =2400 J; 2
=2400 J; 2
Award [2] for correct bald answer.
(ii) (Q =
∆U + W with) ∆U = 0;
so Q = 2400 J; 2
Award [0] for correct answer with no or wrong argument.
(c) (i) curve
under given straight line starting at B and ending at A; 1
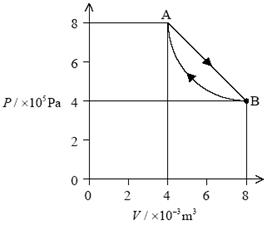
(ii) it would be
less;
since the work done would be less / area under curve is
less (and ∆U = 0); 2
[8]
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.