
Lesson-seminar on "Types of chemical bonds, types of crystal lattices" - structure of MATTER - LESSON DEVELOPMENTS in CHEMISTRY 11 class - lesson developments-lesson developments-author's lessons-plan-lesson summary - chemistry
Lesson objectives: to summarize, consolidate students ' knowledge of the topic and the ability to apply them in solving exercises.
Equipment: tables "Types of chemical bonds", "Types of crystal lattices", crystal lattices (models): ionic, atomic, molecular; codotransporant with questions of theory and practice.
Lesson progress
I. Organizational moment
Setting goals and objectives of the lesson, organizing students to conduct a seminar. Questions of theory are discussed according to their sequence, using notes, text of the textbook, visual AIDS.
II. Discussion of issues of theory and practice
Theoretical part
1. the Concepts of "chemical bond", "electronegativity".
2. The ionic bond. Definition. Examples of the compounds. The mechanism of formation of ionic bonds. Type of crystal lattice, physical properties of substances with an ionic bond.
3. Covalent bonding. Definition. Examples of the compounds. Types of covalent bonding. Mechanisms of covalent bond formation:
a) exchange rate;
b) donor-acceptor;
The types of crystal lattices the physical properties of substances.
4. Metal connection. Definition. Example. Formation mechanism. The type of crystal lattice. Physical property. Similarities and differences with covalent and ionic bonding.
5. Hydrogen bonding. Definition. Examples of the compounds. Mechanisms of hydrogen bond formation. The type of crystal lattice. Physical properties of substances.
6. What is the unity of nature of all types of chemical bonding? Use specific examples to explain the transition of one type of communication to another. Next, we will start discussing the practice questions, which are directly a homework assignment.
Practical part
1. What types of chemical bonds and types of crystal lattices are characteristic of compounds?
N2; CA; CaF2; F2; OF2; K2O2; Na2SO4.
2. Explain the mechanism of bond formation in compounds.
a) N2; b) CA; C) OF2; d) CaF2.
II. Independent work
|
Option I |
Option II |
|
1. Give a characteristic of the compounds according to the plan: the type of bond, the definition, the mechanism of bond formation, the method of overlapping orbitals, the type of crystal lattice, physical properties. |
|
|
a) NF3 b) MgCl2 |
a) H2S b) SL2 |
|
2. Does the compound form a hydrogen bond and what kind of bond is it? |
|
|
HF |
NH3 |
|
Why do they dissolve in water? |
|
III. Summarizing the lesson results
IV. Homework assignment
Repeat: valence States of the carbon atom in organic compounds; hybridization-sp3; -sp2: -sp, entries for the 10th grade, textbook for the 10th grade.
Answers to the theory questions are offered in lesson plans # 1, 2, textbook § 6.
Answers
Practical part
1. Ionic bond-CaF2; ionic crystal lattice. Covalent nonpolar bond — N2; F2; molecular crystal lattice.
Covalent polar bond — OF2; molecular crystal lattice.
CA-metal connection, metal grid.
K2O2— bis connectedby an ionic bond and covalent nonpolar; ionic crystal lattice.
Na2SO4is anии иоionic bond; covalent polar, and in the SO4 aniona 2-ionic crystal lattice.
2. The mechanism of bond formation in joints:
a) N2— covalent, non-polar bond.
+7; 1s22s22p3
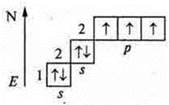
![]() — a
nitrogen atom has a pair of paired electrons and three unpaired by the exchange
mechanism with another nitrogen atom, there is the formation of three common
electron pairs, there is an overlap of p-orbitals, one axial-σ-bond and
two lateral overlaps of the 2π-bond.
— a
nitrogen atom has a pair of paired electrons and three unpaired by the exchange
mechanism with another nitrogen atom, there is the formation of three common
electron pairs, there is an overlap of p-orbitals, one axial-σ-bond and
two lateral overlaps of the 2π-bond.
![]() The
link multiplicity is 3.
The
link multiplicity is 3.
The crystal lattice is molecular.
b) CA-metal, metal bond, in the crystal there are atoms-ions, free electrons; due to their mutual attraction, the bond is carried out.
C) OF2-covalent polar coupling.
+8; 1s22s22p4
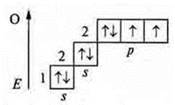
![]() — an
oxygen atom has two pairs of paired electrons and 2p-unpaired electrons.
— an
oxygen atom has two pairs of paired electrons and 2p-unpaired electrons.
-9; 1s22s22p5
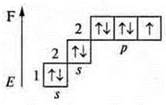
![]() — a
fluorine atom has three pairs of paired electrons and 1 p is an unpaired
electron.
— a
fluorine atom has three pairs of paired electrons and 1 p is an unpaired
electron.
Between the oxygen atom and two fluorine atoms are formed by shared pairs according to the exchange mechanism, because EO EO more fluorine, oxygen, shared e-pair is shifted toward the fluorine atoms.
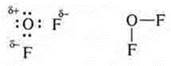
d) the CaF2-ion bond of the compound is formed by elements that differ sharply in EO.
|
CA-metal |
F — nonmetal |
|
CA 4s2 |
F +8; 2s22p5 |
|
|
|
|
gives 2e-atoms of fluorine |
|
|
|
|
|
acquires a charge of +2 |
an atom of fluorine takes one electron, acquires a charge of -1. |
The mutual attraction of oppositely charged particles leads to the formation of a compound with an ionic bond.
![]()
Independent work
Option I
1. a) NF3-covalent polar coupling. Communication by means of common electronic pairs formed by an exchange mechanism
|
N +7; 1s22s22P3 |
F +9; 1s22s22p5 |
|
three unpaired p-electrons |
one unpaired p-electron, three fluorine atoms are required |

The overlap of orbitals is axial, σ-bonds are formed; the multiplicity of the bond between the atoms is 1.
The crystal lattice is molecular, it is possible that it is a gas that easily passes into a liquid.
b) MgCl2-ion bond, a bond due to the electrostatic attraction of oppositely charged particles. The compound is formed by a metal and a nonmetal, which differ sharply in the EO.
|
|
|
The mutual attraction of oppositely charged particles leads to the formation of a compound with an ionic bond:
![]()
Ionic crystal lattice, solid, refractory substance.
2. HF compounds with a polar covalent bond in the molecule have a displacement of the total electron pair to the EO atom of fluorine and a polarity occurs. δ+ - in the case of a hydrogen atom and δ- — in the case of a fluorine atom, fluorine also has non-separated pairs of electrons М, although it is possible to form a hydrogen bond between the molecules.
![]()
Since in the water molecule there is also a shift элекof the total electron pairs to the EO of the atom O-oxygen, which also has unshared pairs of electrons. In a molecule, the hydrogen atoms have a lack of electron density δ+, and the oxygen atom has an excess of δ-.
Between the molecules of hydrogen fluoride and water, the formation of hydrogen bonds is possible, and hydrogen fluoride is soluble in water.
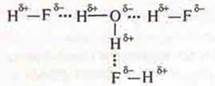
Option II
1. a) H2S-covalent polar coupling, coupling by means of common electron pairs formed by an exchange mechanism.
|
one unpaired s-electron |
at the third energy level two pairs of paired electrons and 2P-unpaired electrons |
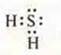
There is an overlap of s-orbitals of hydrogen atoms and p-orbitals of the sulfur atom, the overlap is axial, a σ -bond is formed, mixed in the direction of the EO of the sulfur atom, the multiplicity of the bond is 1; the crystal lattice is molecular.
b) CL2-covalent nonpolar bond, a bond by means of a common electronic pair formed by an exchange mechanism.
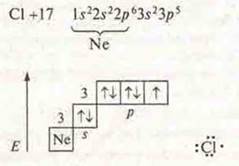
There are three pairs of paired electrons in an atom and one p-electron that is not paired. There is an overlap of p -orbitals, axial, and a σ-bond occurs.
![]()
The multiplicity of the bond is 1. the Crystal lattice is molecular.
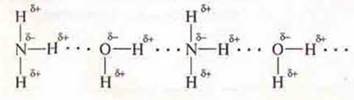
2. NH3 — a compound with a polar covalent bond in the molecule, there is a shift to shared electron pairs EO n atom which has unshared pair of electrons, there is the polarity: δ+ — from the hydrogen atoms, δ- — at the nitrogen atom.
Hydrogen bonds can occur between molecules.
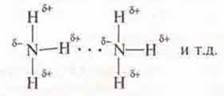
Since in the water molecule, there is also a mixing of common electron pairs to the EO atom-oxygen, which also has non-separated pairs of electrons. Inthe H2 o molecule, the hydrogen atoms have a lack of electron density δ+. the oxygen atom has an excess of δ+.3Hydrogen bonds can form between NH 3 and H2o molecules. NH3is soluble in water.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.