
The universality of the theory of chemical structure of A. M. Butlerov. Modern directions of theory development - structure of MATTER - LESSON PLANS in CHEMISTRY 11 class - lesson plans-lesson plans-author's lessons-plan-lesson summary - chemistry
The purpose of the lesson: to explain the universality of the theory of structure, i.e. its applicability to inorganic compounds; to give an idea of the current trends in the development of the theory of structure and its significance for the development of science and industry.
Equipment: PSHA of D. I. Mendeleyev, the table of the structure of organic compounds.
Lesson progress
I. Organizational moment
Message to students the sequence of work in the lesson. Having studied the main provisions of the theory of structure, it is necessary to prepare monologue answers of students at the blackboard on these questions:
Student 1, 2. the First position of the theory of structure, homework # 5;
Student 2. The third position of the theory of structure, homework, comparison of properties of CH3—NH2; C6H5-NH2;
II. Front work
At the time of preparing students at the blackboard, all other students perform a task on the composition of isomers and determining the type and type of isomerism of the formulas C3H7NO2; C5H10.
Answer: This is an oxygen-containing and nitrogen-containing compound.
Let's assume the formula:
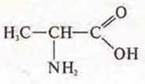 — 2-amino-propanoic acid.
— 2-amino-propanoic acid.
Position
isomer: amino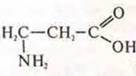 group — 3-amino-propanoic
acid
group — 3-amino-propanoic
acid
Possible
amino group and the nitro group in the compound: 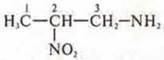 - 3-amino-2-nitropropane
- 3-amino-2-nitropropane
The structural isomer:
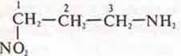 — 3-amino-1-nitropropane
— 3-amino-1-nitropropane
b) S5N10SnN2n: alkene
![]() - penten-1
- penten-1
Isomer at the carbon skeleton:
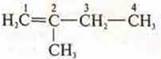 - 2-methylbutene-1
- 2-methylbutene-1
An isomer of position of multiple bonds:
![]() - penten-2
- penten-2
Spatial, geometric:
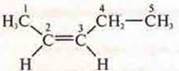 - CIS-penten-2
- CIS-penten-2
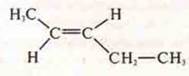 - TRANS-penten-1
- TRANS-penten-1
Interclass isomer:
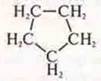 -
cyclopentane.
-
cyclopentane.
III. Checking students ' responses at the blackboard
The students ' answers are checked at theIASC , and the theoretical material is confirmed by the homework.
Student 1. The first and second propositions of the structure theory. Homework # 5.
The types of isomerism and their types are explained by homework.
C3H8O is an oxygen-containing compound.
Perhaps it is alcohol, the functional group-ON.
![]() -
propanol-1
-
propanol-1
Structural isomers:
position of the functional group.
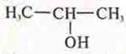 -
propanol-2
-
propanol-2
Interclass isomer: simple ether,
H3S-O-SN2-SN3-methyl-ethyl ether.
Student 2. The third position of the theory of structure.
![]()
The compounds contain an amino group that has a special structure. The nitrogen atom has an unshared pair of electrons, which provides this particle with a large EO.

The basic character of methylamine is much stronger than that of phenyl-amine.
In methylamine, the electron density of nitrogen in amino group 6 is enhanced by the radical-CH3, which contributes to the strong protonation of the hydrogen cation when the substance interacts with water non AND acidHowever, the main properties of phenyl-amine are weakened by the following: an unshielded pair of electrons of the nitrogen atom comes into contact with the p-electron cloud of the aromatic ring, which reduces the electron density, weakens the ability of the substance to protonate a hydrogen cation when interacting with water and acid. The results of checking the homework are summed up, and school points are set.
The universality of the structure theory is the possibility of using its provisions to explain the structure of inorganic substances, the existence of isomers in inorganic substances.
For example:
a) цианатammonium cyanate NH4OCN-an inorganic substance;
urea-CO(NH2)2 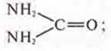
b)
thiourea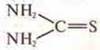 and
ammonium rhodanide H4N—S—C≡N.
and
ammonium rhodanide H4N—S—C≡N.
The phenomenon of mutual influence of atoms in compounds can be considered on the example of hydrogen compounds of non-metals and changes in their properties in periods and groups, the main subgroups. By the end of the period, the acidic nature of hydrogen compounds increases. By the end of the main subgroup group, the main character weakens, and the acidic character increases. Such changes are explained by the different ability of hydrogen compounds of non-metals to break off or attach hydrogen cations in solutions, since in compounds the atom of a non-metal has a different effect on the hydrogen atoms.
Example
1.![]() (compounds
formed by elements of one main group subgroup); and from NH3(ammonia)
main properties were more pronounced than at PH3(phosphine), since
the radius of a phosphorus atom is greater than the radius of a nitrogen atom,
it attracts weaker hydrogen atoms; the charge of the nucleus of an atom of
phosphorus has more nuclear charge of nitrogen atom and he is stronger repels
the hydrogen atom that describes the acidic nature of:
(compounds
formed by elements of one main group subgroup); and from NH3(ammonia)
main properties were more pronounced than at PH3(phosphine), since
the radius of a phosphorus atom is greater than the radius of a nitrogen atom,
it attracts weaker hydrogen atoms; the charge of the nucleus of an atom of
phosphorus has more nuclear charge of nitrogen atom and he is stronger repels
the hydrogen atom that describes the acidic nature of:
Example
2.![]() (connections
are formed by elements of the same period). The acidic properties of HF are
more pronounced than H2O, because the radius of the fluorine atom is
smaller, and the charge of the nucleus is greater than that of oxygen.
whatcauses an increase in the repulsive force of the hydrogen atom
(connections
are formed by elements of the same period). The acidic properties of HF are
more pronounced than H2O, because the radius of the fluorine atom is
smaller, and the charge of the nucleus is greater than that of oxygen.
whatcauses an increase in the repulsive force of the hydrogen atom
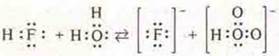
If we consider hydroxides formed by elements of the same period, then there is a change in the acid-base properties of these compounds by the end of the period.
The basic properties of hydroxides decrease, and acidic ones increase, because the degree of oxidation of the Central atom increases, its binding energy with the oxygen atom increases, and the repulsive force of the hydrogen atom increases.
Example
1.![]() - sodium
hydroxide. A stronger bond between an oxygen atom and a hydrogen atom than the
bond between an oxygen atom and a sodium atom. The radius of a hydrogen atom is
smaller than the radius of a sodium atom.
- sodium
hydroxide. A stronger bond between an oxygen atom and a hydrogen atom than the
bond between an oxygen atom and a sodium atom. The radius of a hydrogen atom is
smaller than the radius of a sodium atom.
Dissociation: ![]()
Example
2.![]() -
perchloric acid, the chlorine atom with the largest C. O. and smallest radius
is more strongly bound to oxygen atoms and repels the hydrogen atom more
strongly.
-
perchloric acid, the chlorine atom with the largest C. O. and smallest radius
is more strongly bound to oxygen atoms and repels the hydrogen atom more
strongly.
Dissociation: ![]()
What are the current trends in the development of the theory of the structure of compounds? The following should be noted.
1. Chemical formulas of organic and inorganic compounds can be written not only in structural formulas, but also in semi-structural , abbreviated and electronic ones.
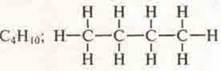 —
structural formula;
—
structural formula;
H3S-SN2—SN2—SN3-semi-structural formula;
H3S—(CH2)2-CH3-abbreviated formula;
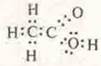 —
electronic formula.
—
electronic formula.
2. Electronic formulas make it possible to explain two effects in organic compounds — inductive and mesomeric.
a) inductive effect — shift of the electron density along the σ-bond system, taking into account the differences in EO.
Example:
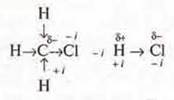
-i — negative inductive effect,
+i — positive inductive effect,
b) mesomeric effect M — displacement of the electron density along the π-bond or displacement of non-separated electron pairs in the presence of alternating Prime and multiple bonds.
The mesomer effect is stronger than the induction effect.
Example:
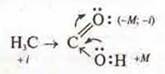
Electronic effects affect the reactivity of the compound, as well as the direction of the process.
Markovnikov's rule: joining hydrogen halide by multiple coupling.
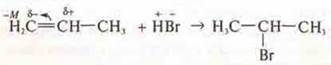
Hydrogen attaches to the most hydrogenated carbon atom at a multiple bond.
It is possible to join contrary to Markovnikov's rule due to the effects of M and i.
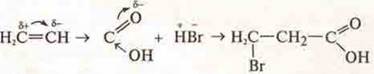
3. The development of the doctrine of spatial isomerism — stereochemistry.
This idea was expressed in 1874 by the French chemist J. A. M. Bel and the Dutch chemist L. X. Vanthoff. Their assumptions are confirmed by quantum chemistry. The property of substances is influenced by the spatial structure of their molecules: qiC- and TRANS-isomers. The process of applying a spatial isomer requires a large amount of energy. It is not possible under normal conditions невозможен. There are many examples of spatial change in the synthesis of IUD (high-molecular-weight compounds). The synthesis of stereoregular rubbers consisting only of CIS-links is underway, which provides high elasticity. Therefore, it is necessary to Supplement the second position of the theory of the structure of compounds: the properties of substances depend not only on their qualitative and quantitative composition, but also on their chemical, electron-spatial structure.
4. thanks to the theory of the structure of compounds, organic chemistry has turned from a descriptive science to a creative one.
Currently, it is possible to synthesize substances with predetermined properties, predict the isomerism of substances, and describe the directions and flow of synthesis reactions.
Thanks to the theory of structure, many substances have been created that replace natural polymers, dyes, and proteins needed in engineering, everyday life, medicine, and agriculture.
The significance of A. M. Butlerov's theory of the structure of compounds for organic chemistry is undeniable, as is the Periodic law and Periodic system of chemical elements of D. I. Mendeleev for inorganic chemistry. Both theories have a lot in common in the ways of their formation, the direction of development and scientific knowledge.
Generalization and conclusions on key issues.
IV. Homework assignment
§ 9, questions # 1, 2, 3 orally.
Compare: 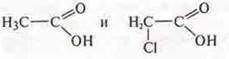
Predicted
substance: 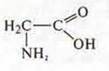
V. Pinning
Using the structural formula, analyze the displacement of electron density, reaction centers, and assume chemical activity:
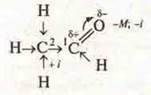
C11+ 1 intermediate C2+ 3 atomscan be oxidized.
1) there is a double bond between C and O: σ - and π-the joining reaction is possible;
2) due to the polarization of the h atom in CH3, a substitution reaction is possible;
3) the oxidation reaction, since the most vulnerable h atom in the aldehyde group.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.