
Credit on the topic "Chemical reactions" - CHEMICAL REACTIONS - LESSON PLANS for CHEMISTRY class 11 - lesson plans - lesson plans-author's lessons-plan-lesson summary - chemistry
Lesson objectives: to check the level of theoretical knowledge of the topic and the ability to apply it in performing calculation tasks and exercises.
Equipment: theory and practice task cards for I—II variants.
Lesson progress
I. Organizational moment
Instructions for completing the test.
II. Standings
Option 1
1. Determination of the speed of a homogeneous chemical reaction, formula of the unit of measurement. How the speed of a chemical reaction depends: a) on the concentration; b) on the catalyst. Give a reasonable answer.
2. To characterize the chemical reactions by all indications, classification.
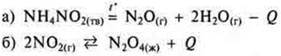
3. According to the thermochemical equation H2+ VG2= 2hvg + 66.8 kJ calculate the mass of bromine that reacts, if 3.34 kJ of heat is released.
4. In which direction will shift the chemical equilibrium of the following system:
![]()
a) when the temperature drops; b) when the pressure increases.
Option II
1.
Determination of chemical equilibrium. The constant of chemical equilibrium.
How changes in the concentration of substances and temperature affect the shift
of chemical equilibrium. Give a reasonable response based on the example of the
reaction ![]()
2. To characterize the chemical reactions are all signs of classification:
![]()
3.
How will the rate of chemical reaction change![]() if the
pressure is increased by 4 times?
if the
pressure is increased by 4 times?
III. Summarizing the lesson results
Answers to practice questions
Option I
1. The question of theory.
2. ![]()
decomposition reaction:
the reaction IAD

the reaction of heterogeneous — phase;
endothermic reaction - by thermal effect;
the reaction is irreversible, non-catalytic.
b) ![]()
the reaction of isomerization;
the reaction is not an IIA;
phase — heterogeneous reaction on thermal effect: exothermic, the reaction is reversible, non-catalytic reduction.
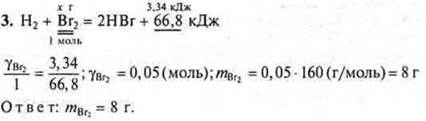
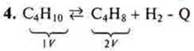
a) t°↓ in the direction of the exothermic <reverse.
b) P↑ in the direction of a smaller volume of information.
Option II
1. The question of theory.
![]()
reaction of a compound;

homogeneous reaction-by phase;
endothermic reaction - by thermal effect;
the reaction is reversible;
the reaction is non-catalytic.
![]()
exchange reaction; heterogeneous reaction-by phase;
the reaction is not OVR; the reaction is non-catalytic;
the reaction is irreversible; the thermal effect is not specified.
![]() P↑
by 4 times
P↑
by 4 times
until
the pressure increases: ![]()
![]()
after
increasing the pressure: ![]()
![]()
![]()
Answer: the reaction speed will increase 64 times.
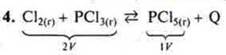
a) P↑ towards a smaller volume, → (straight line);
b)![]() →
(straightline) towards the final products of the reaction.
→
(straightline) towards the final products of the reaction.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.