
Dissolution. Solubility. Quantitative characteristics of solutions-DISPERSED SYSTEMS. SOLUTIONS. PROCESSES OCCURRING in SOLUTIONS - LESSON DEVELOPMENTS in CHEMISTRY grade 11 - lesson developments-lesson developments - author's lessons - lesson plan-lesson summary - chemistry
Lesson objectives: to clarify the meaning of the concept "solubility" and find out how it differs from the concept of "mass fraction of solute"; tell about ways of expressing concentrations of substances; to teach to apply knowledge of the concepts of "solubility", "concentration of solutions" when solving design problems.
Basic concepts: solubility, solubility coefficient, saturated solution, unsaturated solution, mass fraction of solute, percentage concentration, molar concentration, volume fraction, molar fraction.
Equipment: CuSO4(anhydrous), CuSO4· 5H2O; H2O, thermometer, tableware.
Lesson progress
I. Interview with students on key issues.
1. Define a dispersed system, a dispersed phase, and a dispersed medium.
2. Name the characteristics of classification of dispersed systems; give examples.
3. What is the value of a disperse system are in nature and in everyday life?
4. To define solution, solvent. What types of solutions are known?
5. is Dissolution a physical or physico-chemical process? Substantiate the interaction of anhydrous copper (II) sulfate with water on the basis of an experiment.
In the course of the conversation with students, the theoretical knowledge of the question of dispersed systems is being consolidated. Students find answers to questions in their notes and textbooks.
In the course of discussing the latter question, an experiment is carried out that proves that dissolution is a physical and chemical process.
Along with the uniform distribution of particles of the dispersed phase between the particles of the dispersion medium, there is a physical process, diffusion. There is an interaction between them, which proves the release of heat and color change, and these are signs of a chemical reaction.
![]()
The solution contains not only solute particles, solvent particles, but also the product of their interaction-hydrated copper (II) cations, which give the solution a blue color. It should be noted that a covalent bond is formed by the donor-acceptor mechanism between the copper (II) cation and water molecules, as a result of which heat is released.
II. Learning new material
Plan of presentation
1. Solubility. Solubility coefficient. Table of solubility of salts, alkalis, and acids in water.
2. Solutions saturated, unsaturated.
3. Quantitative characteristics of the solution composition:
a) molar concentration of the solution;
b) mass fraction of the dissolved substance — the percentage concentration of the solution;
C) volume and molar fractions of the solute.
4. Algorithm for solving calculation problems for quantitative characteristics of solutions.
What is the solubility of substances? This is the ability of substances to break down to structural units under the action of a solvent. Solubility depends on the nature of the solute, the nature of the solvent, temperature, pressure for gases. By solubility, substances are divided into well-soluble (dissolves more than 1 g in 100 g of solvent), poorly soluble (dissolves from 0.01 g to 1 g in 100 g of solvent) and practically insoluble substances (dissolves less than 0.01 g in 100 g of solvent at a temperature of 25°C). SSat. t°— solubility of the substance at a certain temperature.
However, at a different temperature, the solubility of the substance is different. Therefore, the solubility coefficient is applied. It shows how many grams of a substance can be dissolved in 100 g of solvent at a given temperature.
Example: At t° = 30 °C in 100 g of solvent dissolves CuSO430 g, and at t° = 70 °C 50 g CuSO4(the last reference provides a determination of solubility in 1000 g solvent).
Solutions can be unsaturated and saturated. A saturated solution contains the maximum amount of solute at a given t°. An unsaturated solution contains less solute at a given t° than it can dissolve.
To Express the composition of a solution, quantitative characteristics of solutions or concentrations are used — molar concentration of the solution; mass fraction of the solute or percentage concentration.
The molar concentration of a substance indicates the amount of moles of a substance in 1 l of solution:
![]()
Mass fraction of solute indicates the amount of solute in 100 g of solution:
![]() (percent
concentration).
(percent
concentration).
If there are gases in the system, then the volume fraction-φ is applied:
![]()
Sometimes it is necessary to calculate the molar fraction of the solute.
![]()
Further, students are offered calculation tasks for quantitative characteristics of solutions.
III. Solution of calculation problems for quantitative characteristics of solutions
Some recommendations should be kept in mind when solving tasks:
a) if V (ml) — volume, ρ (g/ml) — density and Wp.n. are known in the condition of the problem, the mass of the solution should first be determined using density and volume: mp-RA= p · V and entered into the formula of the mass fraction of the solute:
![]()
b) if it is necessary to determine the mass fraction of a dissolved gaseous substance, it is necessary to determine the volume of the quantity of the substance, and then its mass:
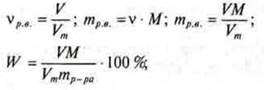
C) determination of the mass fraction of the dissolved substance, if the solubility is known:
![]()
Problems with the concept of "solubility»
No. 1. The solubility of sodium bromide at 20 °C is 905 g. What mass of salt can be dissolved in water weighing 900 g at 20 °C?
Given:
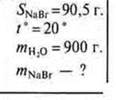
I method
II method
in 100 g of water at 20° 90,5 g is dissolved
and in 900 g of water - ” - x g
![]()
Answer: you can dissolve 814.5 g of NaBr.
No. 2. in what mass of water is it necessary to dissolve potassium nitrate weighing 165 g to get a saturated solution at t° = 35 °C? SKNO3= 75 g; t° = 35 °C (75 g is dissolved in 100 g of water at t° = 35°).
Given:
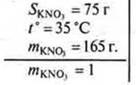
I method
In 100 g of water at 35°, 75 g is dissolved
In x g of water at 35 ° — " - 165 g
X = 220 g of water
II method
mof saturated solution= 100 g + 75 g = 175 g
175 g — 75 g of salt
Xus.R-RAg-165 g of salt
x = 385 g-the mass of the saturated solution.
![]()
Answer: mH2O= 220 g.
Tasks on the composition of a solution with a mass fraction of solute
No. 1. Calculate the mass of iodine and alcohol required for the preparation of a solution weighing 300 g, Wp. V.= 10%.
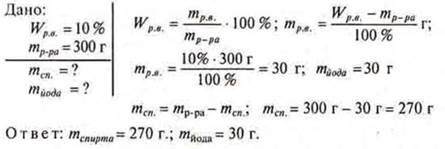
Tasks for determining the molar concentration of a solution
The 500 ml solution contains 8 g of sodium hydroxide. Determine the molar concentration of the solution.
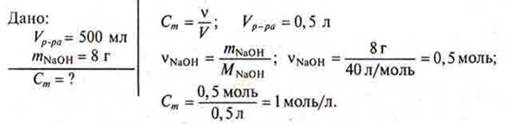
Tasks for the transition from one expression the concentration of the solution in the other
When solving such problems, it should be remembered that the mass of the solute is the same. First, you should Express the mass of solute for each type of concentration, and then equate these expressions.
Example: we know the mass fraction of dissolved substance W.It is necessary to convert this concentration to a molar WithM.
1) Write down the formula for expressing these concentrations and Express the mass of the solute in both cases:
![]() CMis
the molar concentration of the solution.
CMis
the molar concentration of the solution.
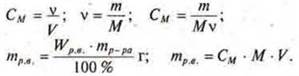
2) Since the mass of the solute in both cases is the same, we write:
![]()
3) we Express the molar concentration of a substance
![]()
Task. Find the molar concentration of hydrochloric acid solution R = 1.19 g / ml with WHCl= 36%.
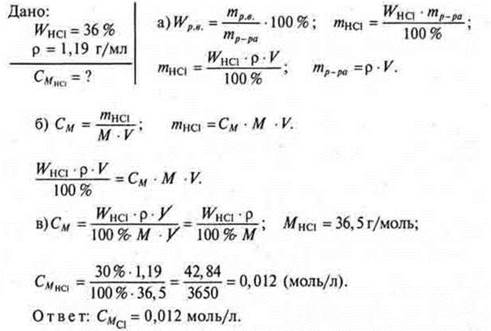
Solving problems in the case of dilution, mixing, and concentration of solutions
No. 1. To 250 g of glucose solution W = 10% is added 150 ml of water. КаковаWhat is the glucose concentration in the new solution?
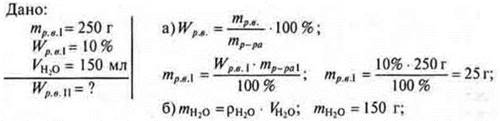
C) Since only mR-RA changed, mglucoseremained unchanged.
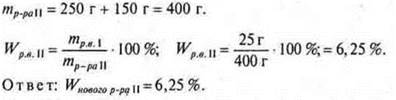
# 2. What mass of salt should be added to the solution of salt weighing 300 g, WR. V.= 20%, to get a solution of WR. V.= 40%?
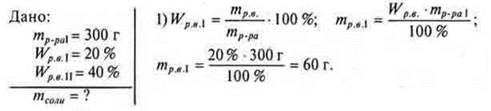
2) Let the mass of salt to be added be x g.
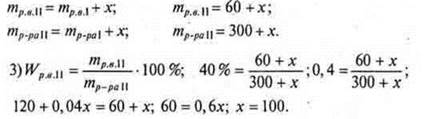
Answer: you should add 100 g of salt.
IV. Homework assignment
§ 8, records.
Solve problems according to the following conditions:
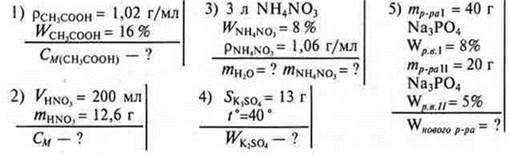
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.