
Control work # 4 on the topic "Substances and their properties" - SUBSTANCES AND their PROPERTIES - LESSON PLANS in CHEMISTRY 11 class-lesson plans-lesson plans-author's lessons-plan-lesson summary - chemistry
Lesson objectives: check the level of comprehension of the topic's key questions.
Equipment: tests of 2 levels of difficulty.
Lesson progress
I. Organizational moment
Instruction on the implementation of control work on options.
II. Completing a test paper
Option
1. Атомwhat element Atom has an electronic configuration of the atom: 1S22S22P63S23P64S1? Write down the formula of its oxide and hydroxide, specify their nature. Draw up equations of reactions (at least two) confirming their chemical properties.
2. Make up the reaction equations of the transformation scheme:

3. Make a genetic series of substances, write reaction equations, specify the names of substances and conditions for the flow of chemical reactions:
4. Draw up the IB interaction equationsCA with H2SO4(K) and C with H2SO4(K).
5. To solve the problem:
What mass of zinc sulfate can be obtained by reacting 13 g of zinc with a solution of sulfuric acid weighing 245 g, WH2SO4= 10 %.
Answers to test questions
1. 1s22s22p63s23p64s1.
The fourth energy level ⇒of the fourth period element has been opened; at the external level, there is one s-electron ⇒of the first group, since it is the s-element ⇒of the main subgroup. This is potassium K.
Potassium
oxide — salt-![]() forming,
basic oxide; potassium hydroxide-CON-base, oxygen-containing, monoacid,
soluble, non-volatile, strong, stable.
forming,
basic oxide; potassium hydroxide-CON-base, oxygen-containing, monoacid,
soluble, non-volatile, strong, stable.

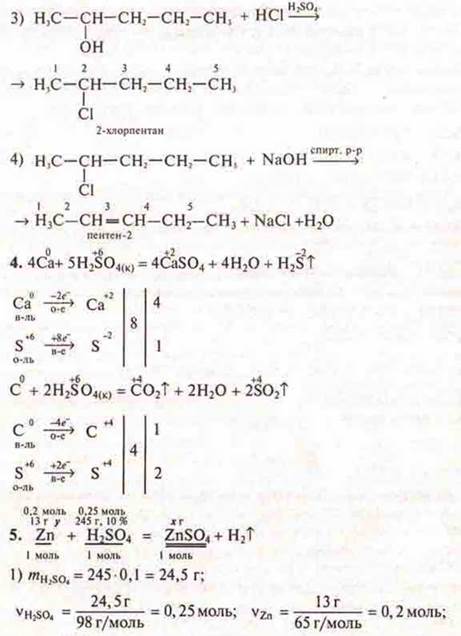
2)![]() y
= 25 mol Zn is required, and given 0.2 mol ⇒
× Zn is insufficient. The calculation is based on a lack.
y
= 25 mol Zn is required, and given 0.2 mol ⇒
× Zn is insufficient. The calculation is based on a lack.
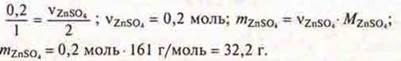
Answer: mZnSO= 32.2 g.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.