
Laboratory work № 13
Investigation of the properties of unsaturated carboxylic acids
The purpose of the work: to get acquainted with the physical and chemical properties of unsaturated carboxylic acids.
Reagents and equipment: amyl alcohol, concentrated sulfuric acid, oleic acid, 5% solution of potassium permanganate, 10% solution of sodium hydroxide, ethyl alcohol, Caci2 solution, test tubes, tripod, alcohol lamp, separation funnel, water bath, gas tube, flask, glass, ice or snow.
Experience the №1. Substances formed when acids are heated
A few crystals of coumysic acid are placed in a test tube and heated. In the upper part of the test tube, keep the paper soaked in lime (barite) water. What did you notice?
№2-practice. Oxidation of oleic acid
Progress: add 1 ml of 5% potassium permanganate solution 1 ml of 10% sulfuric acid solution and 0.5 ml of oleic acid to the test tube. Beat the mixture in a test tube and mix. What did you notice?
The reaction equation:
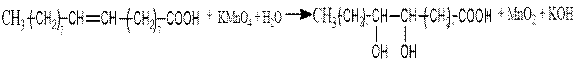 9,10- dioxystearic acid
9,10- dioxystearic acid
№3-practice. Preparation of oleic acid salt
Progress: 2 drops of oleic acid and 4-6 drops of distilled water are poured into the test tube. Mix thoroughly, add 6-8 drops of alkaline solution. The resulting mixture is heated in an alcohol lamp. Sodium water-soluble
when whipping, a foam is formed due to the formation of soap. Drop 2-3 drops of calcium chloride solution in the resulting soap solution. Pay attention to the white tincture of calcium soap, insoluble in water.
№4-practice. Interaction of oleic acid with alkali. Purchase of soap
Progress: 2 ml of 10% alkali solution is poured into the test tube, 2-3 drops of oleic acid are added. The mixture is strongly mixed. Then the soap bubble adds oleic acid. The mixture is strongly mixed. At that time, the formation of a soap bubble is observed. This is the sodium salt of oleic acid (soap).
С17Н33СООН + NaOH ® C17H33OONa + H2O
Experience №5. Isomerization of oleic acids into elaidin.
Reagents and equipment: oleic acid, copper (diameter 0.5-1 mm).
In a test tube, pour 2 ml of oleic acid and place a purified copper wire (0.1-0.2 g), which includes 1 ml of concentrate. HNO3 adds. The tube is capped and simply shaken so that the nitrogen oxides are well absorbed into the oleic acids as a result of the interaction of nitric acid with copper.
The mixture in the test tube is heated, opened and closed with a stopper to avoid increasing the pressure of the mixture in the test tube. After a few minutes, when the bubbles of the released gas stop, the test tube is tightly closed and left on a tripod. After about 1 hour, the oleic acid layer on the surface of the mold of the water-acid layers thickens or compacts strongly, and does not spill when the test tube is tipped over.
Questions for self-monitoring and protection of laboratory work:
1. write the reaction equations for the inclusion of bromine in oleic acid
2. Write the reaction of sodium soap formation.
3. write the reaction of calcium soap formation.
4. Write the equation for the reaction of loss of Koumiss acid
5. write the reaction equation between the product formed by the decomposition of koumiss acid from lime (barite) water.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.