
Heat and thermodynamics.
Learning objective
10.2.2.1 - apply the ideal gas equation of state and distinguish gas process graphs;
10.2.3.1-explain the meaning of the first and second laws of thermodynamics
10.2.3.2-describe the operation and application of the heat engine;
10.2.4.1-determine relative humidity;
10.2.4.2-explain the nature of surface tension and the role of capillary phenomena in everyday life;
PART1: MULTIPLE CHOICE QUESTIONS
Instructions:
· For each question there are five possible answers A, B, C, D and E. Choose the one you consider the best and correct.
· Mark your answers to multiple choice questions in the space provided below.
Begin Here:
1. An ideal gas in a cylinder with a movable piston is made to follow the process graphed by the path A→B→C→A. What work done of the gas?
1.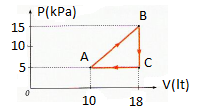
A 20 J B 40 J C 60 J D 80 J
________________________________________________![]() ______[1]
______[1]
2 What is the rms speed of nitrogen molecules contained in 6.4 m3 volume at 4.2 atm if the total amount of nitrogen is 1800 mol?
A 100 m/s B 200 m/s C 400 m/s D 800 m/s
________________________________________________![]() ______[1]
______[1]
3.Thirty joules of heat flow into a system. The system in turn does 50 joules of work. The internal energy of the system has A increased by 80 J B decreased by 80 J С increased by 20 J D decreased by 20 J
________________________________________________![]() ______[1]
______[1]
4. A gas is taken from an initial state to a final state as shown. How much work is done during this
process?
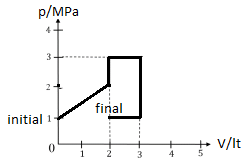
A 5 kJ B 6 kJ C 7 kJ D 8 kJ
________________________________________________![]() ______[1]
______[1]
5. An ideal gas absorbs 750 J of heat in the process of an isobaric expansion. What is the resulting change in temperature if there are 3 moles of the gas in the system?
A 12 K B 16 K C 20 K D 24 K
________________________________________________![]() ______[1]
______[1]
6. Which are graphs represents an isochoric process
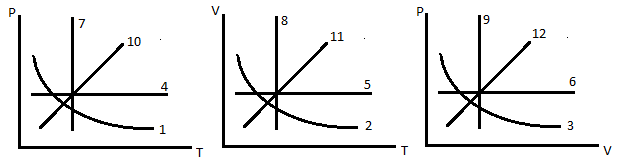
A 5,9,10 B 5,6,10 C 4,8,9 D 5,9,12
________________________________________________![]() ______[1]
______[1]
7.What is the relative humidity for following readings?
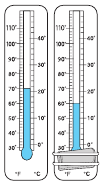
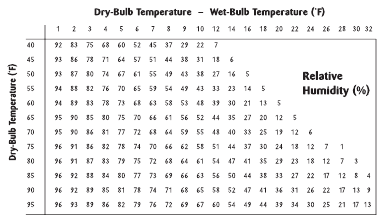
A 48% B 52% C 55% D 58%
________________________________________________![]() ______[1]
______[1]
8.Which can increase the relative humidity?
A increase air temperature B decrease air temperature C increase dew point D decrease dew point
________________________________________________![]() ______[1]
______[1]
9.Water rises to a height of 20 mm in a capillary. If the radius of the capillary is made 1/3 rd of its previous value, to what height will the water now rise in the tube?
A 40 mm B 60 mm C 80 mm D 90 mm
_______________________________________________________![]() ______[1]
______[1]
10.Water rises in a glass capillary tube to a height of 9 cm. What is the diameter of the capillary tube? Surface tension of 72.0 mN/m.
A 0.08 mm B 0.16 mm C 0.32 mm D 0.48 mm
_______________________________________________________![]() ______[1]
______[1]
PART 2: SHORT-ANSWER QUESTION
Instructions:
· Read each question carefully.
· Answer all the questions.
· Show all calculations and write your answer in the space provided.
11. A system of monatomic ideal gas contains a certain number of moles of gas so that nR=0.200 J/K . The system is taken from state 5 to state 6 (see Figure 15.25) by an unknown sequence of processes.
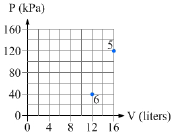 a. What is the temperature of
the system in state 6?
______[1]
b. What is the work
done by the gas as the system moves from state 5 to state 6?
_____[1]
c. What is the change in internal energy of the gas as
it moves from state 5 to state 6?_________[1]
a. What is the temperature of
the system in state 6?
______[1]
b. What is the work
done by the gas as the system moves from state 5 to state 6?
_____[1]
c. What is the change in internal energy of the gas as
it moves from state 5 to state 6?_________[1]
12. An engine absorbs 1.60 kJ from a hot reservoir and expels 1.20 kJ to a cold reservoir in each cycle.
a. What is the engine’s efficiency?__________[1] b. How much work is done by the engine in each cycle?_________[1] c. What is the power output of the engine if each cycle lasts 0.25 s?______[1]
13. 2500 J of heat is added to a system, and 1800 J of work is done on the system. What is the change in internal energy of the system? _________[2]
14. A steam engine operates between 500°C and 270°C. What is the maximum possible efficiency of this engine?_________[2]
15. A mercury drop of radius 10-3 m breaks up into 125 small droplets. Calculate the change in energe assuming that the drops are spherical and surface tension of the mercury is 0.55 N/m. ____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________[4]
16. A needle 5 cm long can just rest on the surface of water without wetting. What its weight? Surface tension of water = 0.07 N/m. _________[2]
PART 3: LONG ANSWER QUESTIONS
Instructions:
· Read each question careful.
· Answer all the questions.
· If the question requires calculation, show all work and your complete solution.
17. The chart shows the change of state of the ideal gas in coordinate plane pressure against temperature. 1-2-3-1
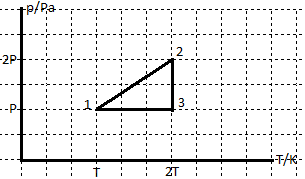
a.Draw new charts to show changes of state of the ideal gas in coordinate planes the pressure against volume and the volume against temperature
 _____________________[3]
_____________________[3]
 ______________________[3]
______________________[3]
To determine the amount of heat in each process, if n=3 moles and T= 400 K
__________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________[6]
To find a job in the cycle _______________________________________________________________________________________________________________________________________________________________________________________________________________________________[2]
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.