
Fundamental principles of molecular-kinetic theory:
1. Which one of the following is not an assumption about the properties of particles in the simple kinetic theory?
|
A |
< c2 > is the average speed of the particles |
|
B |
The forces between the particles are negligible except when particles collide |
|
C |
The time spent by particles in collision is negligible compared with the time spent between collisions |
|
D |
The volume of the particles is negligible compared to the volume of the container |
2. The diagram below shows a number of smoke particles suspended in air. The arrows indicate the directions in which the particles are moving at a particular time.
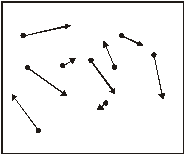
(a) (i) Explain why the smoke particles are observed to move.
(ii) Smoke particles are observed to move in a random way. State two conclusions about air molecules and their motion resulting from this observation.
(b) A sample of air has a density of 1.24 kg m–3 at a pressure of 1.01 × 105 Pa and a temperature of 300 K.
the Boltzmann constant = 1.38 × 10–23 J K–1
(i) Calculate the mean kinetic energy of an air molecule under these conditions.
(ii) Calculate the mean square speed for the air molecules.
(iii) Explain why, when the temperature of the air is increased to 320 K, some of the molecules will have speeds much less than that suggested by the value you calculated in part (b)(ii).
3. In stars, helium-3 and helium-4 are formed by the fusion of hydrogen nuclei. As the temperature rises, a helium-3 nucleus and a helium-4 nucleus can fuse to produce beryllium-7 with the release of energy in the form of gamma radiation.
The table below shows the masses of these nuclei.
|
Nucleus |
Mass / u |
|
Helium-3 |
3.01493 |
|
Helium-4 |
4.00151 |
|
Beryllium-7 |
7.01473 |
(a) (i) Calculate the energy released, in J, when a helium-3 nucleus fuses with a helium-4 nucleus.
energy released ____________________ J
(ii) Assume that in each interaction the energy is released as a single gamma-ray photon.
Calculate the wavelength of the gamma radiation.
wavelength ____________________ m
(b) For a helium-3 nucleus and a helium-4 nucleus to fuse they need to be separated by no more than 3.5 × 10–15 m.
(i) Calculate the minimum total kinetic energy of the nuclei required for them to reach a separation of 3.5 × 10–15 m.
total kinetic energy ____________________ J
(ii) Calculate the temperature at which two nuclei with the average kinetic energy for that temperature would be able to fuse.
Assume that the two nuclei have equal kinetic energy.
temperature ____________________ K
(c) Scientists continue to try to produce a viable fusion reactor to generate energy on Earth using reactors like the Joint European Torus (JET). The method requires a plasma that has to be raised to a suitable temperature for fusion to take place.
(i) State two nuclei that are most likely to be used to form the plasma of a fusion reactor.
1.
2.
(ii) State one method which can be used to raise the temperature of the plasma to a suitable temperature.
Fundamentals of thermodynamics:
4. The figure below shows a child’s ‘pop’ gun in which a piston is pushed quickly along the barrel, compressing the air in the barrel. When the pressure is high enough, the cork is expelled at high speed from the end of the barrel.
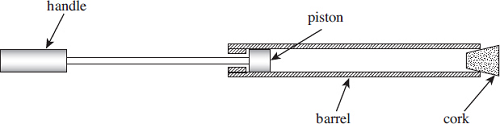
The
figure above shows the gun before it is ‘fired’. The air in the barrel is at a
pressure of
1.0 × 105 Pa, a temperature of 290 K and the volume is 2.1
× 10–5 m3.
(a) (i)
The volume of air in the barrel at the instant the cork is expelled is 1.2
× 10–5 m3.
Calculate the pressure of the air in the barrel at the instant the cork is
expelled.
Assume that the air is compressed adiabatically.
adiabatic index, γ, for air = 1.4
answer = ____________________ Pa
(ii) Calculate the maximum temperature reached by the air in the gun. Give your answer to an appropriate number of significant figures.
answer = ____________________ K
(b) The work needed to compress the air adiabatically from 2.1 × 10–5 m3 to 1.2 × 10–5 m3 is 1.4 J. Use the first law of thermodynamics to determine the change in internal energy of the air during the compression. Explain how you arrived at your answer.
answer = ____________________ J
(c) Explain, giving your reasons, whether the volume of air in the barrel at the point when the cork leaves the gun would be less than, equal to, or greater than 1.2 × 10–5 m3 if the handle of the gun had been pushed in slowly. Assume there is no leakage of air past the cork or piston. You may find it helpful to sketch a p - V diagram of the compression.
Liquid and solid bodies:
5. What must be the elongation of a wire 5m long so that the strain is 1% of 0.1? If the wire has cross-selection of 1mm² and is stretched by 10 kg-wt, what is the stress?
6. A brass wire of length 2 m has its one end, fixed to a rigid support and from the other end a 4 kg wt is suspended. If the radius of the wire is 0.35 mm, find the extension produced in the wire. g = 9.81m/s² ,Y = 11 × 1010 N/m²
7. A wire of length 1.5 m and of radius 0.4 mm is stretched by 1.2 mm on loading. If the Young’s modulus of its material is 12.5 × 1010 N/m². , find the stretching force.
Gas laws:
8. The figure below shows a closed cyclic process on the coordinate axes V-T for a constant mass of an ideal gas.
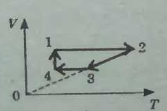
a Name each section of this cycle, and for each section identify the process that is occurring: compression, expansion, heating, or cooling.
b Draw this cycle on coordinate axes p-V.
с Draw this cycle on coordinate axes p-T.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.