
Solving problems (solutions and answers)
1) Isothermal process => T = const
P1·V1 = P2·V2
V2 = ![]()
V2 = ![]()
V2 = 50 m3
The gas will expand, but the pressure will decrease
P(V) graph
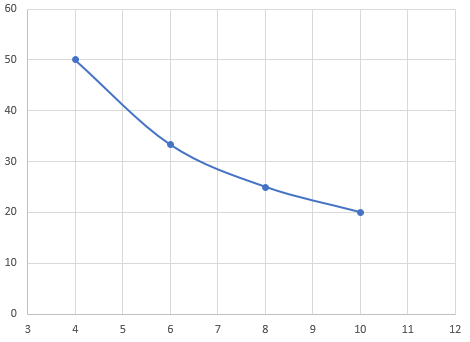
V(T) graph
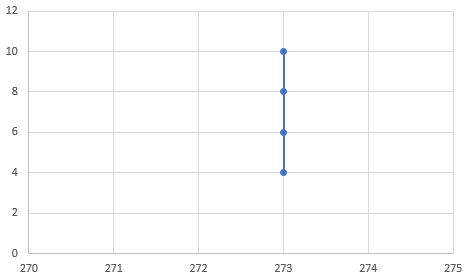
P(T) graph
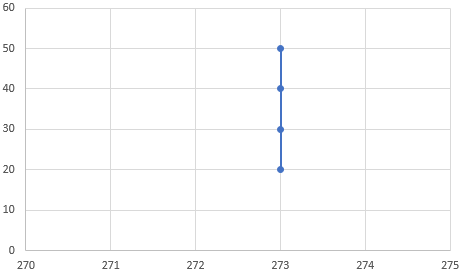
2) Isobaric process => P = const
![]() =
= ![]()
V2 = V1·![]()
V2 = 1 m3·![]()
V2 = 1.5 m3
The gas will expand as its temperature increases
P(V) graph
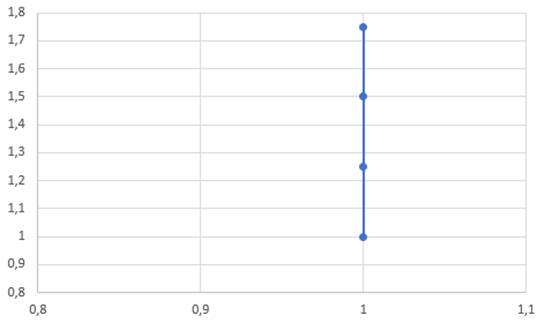
V(T) graph
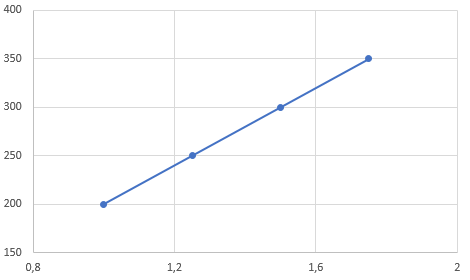
P(T) graph
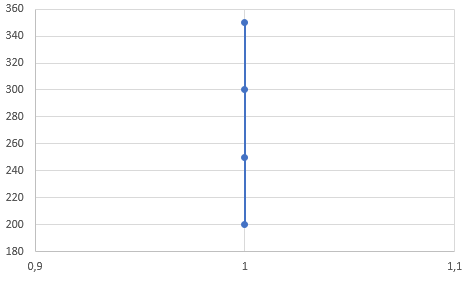
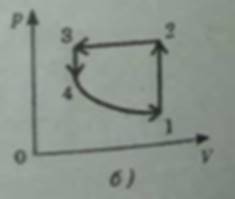
3) a) 1 → 2 isochoric heating
2 → 3 isobaric compression with cooling happening
3 → 4 isochoric cooling
4 → 1 isothermal expansion
P(V) graph
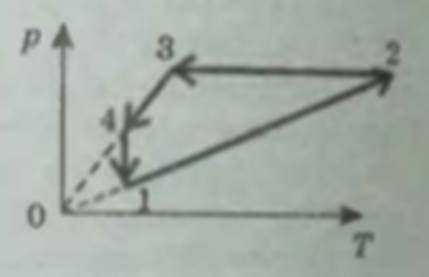
P(T) graph
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.