
1. When the pressure of an ideal gas of a given mass at a temperature of Т=273 K is P1=10 kPa its volume V1=20 m3.
Construct a graph of the isothermal process of this gas if the final gas pressure P2=4 kPa.
a on coordinate axes p-V.
b on coordinate axes V-T.
c on coordinate axes p-T.
2. In its initial state, a gas occupies a volume V1=1 m3 at a temperature of Т1=200 K. The final temperature of the gas is Т2=300 К.
Construct a graph of the isobaric process of the ideal gas at a pressure of P=1·105 Pa:
a on coordinate axes p-V.
b on coordinate axes V-T.
c on coordinate axes p-T.
3. The figure below shows a closed cyclic process on the coordinate axes V-T for a constant mass of an ideal gas.
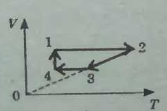
a Name each section of this cycle, and for each section identify the process that is occurring: compression, expansion, heating, or cooling.
b Draw this cycle on coordinate axes p-V.
с Draw this cycle on coordinate axes p-T.

Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.