
Investigation sheet
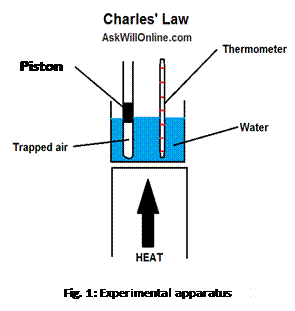 Charles’ Law: A
group of Physics students wish to investigate how the volume of a fixed mass of
an ideal gas depends on the temperature of the gas, at constant pressure. To do
so, they carry out an experiment using the apparatus shown in fig. 1.
Charles’ Law: A
group of Physics students wish to investigate how the volume of a fixed mass of
an ideal gas depends on the temperature of the gas, at constant pressure. To do
so, they carry out an experiment using the apparatus shown in fig. 1.
1. a) State the dependent and independent variable for this experiment.
Dependent variable ……………………………………………………………………………
Independent variable ………………………………………………………………………… [1]
b) Why is it important to keep pressure constant when investigating the dependence of volume on temperature?
…………………………………………………………………………………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………………………………………….…………………………….. [1]
c) Which condition must be verified in fig.1 to ensure that the gas expands at constant pressure?
…………………………………………………………………………………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………………………………………….…………………………….. [1]
2. The students’ results were recorded on the following table.
|
t/oC |
V/mL |
|
0 |
114 |
|
25 |
124 |
|
50 |
134 |
|
75 |
145 |
|
100 |
155 |
a) Use these results to plot a graph of volume V on the y-axis against temperature t on the x-axis.
[3]
b) Draw the line of best fit on your graph and find the gradient k of that line. Show clearly, on the graph, how you worked this out. Show your work.
k = ………………………………………………. [4]
c) State the y-intercept b and the mathematical equation of the line of best fit in the form y = kx + b.
b = ………………………………………………
Equation = …………………………………………………………. [1]
d) Determine at which temperature the volume of the gas reduces to zero (hypothetical situation).
Show your work.
t = ……………………… oC [2]
e) State the name that is commonly used in Physics for value found in question 2. d).
……………………………………………………………………………………………………………………………………………………………………….…………………………….. [1]
3. a) The air in fig. 1 is trapped inside a non-graduated cylindrical test tube. Suggest and describe a way to measure the volume of the gas as it expands.
……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………………………………………….…………………………….. [3]
b) Students use an electric hot plate, whose power can be controlled, to heat the water. In this way, students can control how fast the water is heated.
Discuss the sources of uncertainty and/ or limitations when using
i) a high power (fast heating);
…………………………………………………………………………………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………………………………………….…………………………….. [1]
ii) a low power (slow heating).
…………………………………………………………………………………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………………………………………….…………………………….. [1]
c) On this experiment, water is heated until it boils. State two safety precautions to be taken by the students.
…………………………………………………………………………………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………………………………………………….…………………………….. [1]
[Total: 20]
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.