
Қостанай ауданы әкімдігі білім бөлімінің «Тобыл қаласының №1 орта мектебі» ММ
ГУ «Cредняя школа №1 города Тобыл» отдела образования акимата Костанайского района
SI «Tobyl secondary school №1» education department of Kostanay district akimat

SOLUTION OF CALCULATION PROBLEMS IN CHEMISTRY WITH ECOLOGICAL CONTENT
Methodical development
Савенко Наталья Борисовна
Учитель химии и биологии
An exercise book for students:
Respected guys. Here is an unusual problem book of calculation and qualitative problems in chemistry. Here you will find typical tasks of the school chemistry course, but in the conditions of the tasks you will find information that will help you think about the problems associated with the preservation and promotion of health. Solving problems, you will learn a lot about the practical application of chemical substances, how to use the knowledge of physical and chemical properties in practice. In the second part of the task book (non-Standard tasks) there are materials that will help you try your hand at compiling such tasks and, if desired, make your own mini-task book. The problem book will be interesting to teachers of chemistry. The material provided in this manual will also help students in preparing for school chemistry Olympiads. This material can also be used in the classroom when solving computational problems in chemistry.
I. Computational and qualitative problems.
1. Basic concepts of chemistry.
Any science has its own terminology, without the assimilation of which the further study of the subject of chemistry will be a serious problem. Knowledge of concepts such as: atom, chemical element, molecule, chemical formula, mass fraction, mole, equations of chemical reactions, coefficient, index and others, you need not only in the study of chemistry. They operate physicists, biologists, ecologists, mathematicians. We offer to test your knowledge
1.1. Concepts: substance, body, chemical and physical phenomena, chemical element, signs of chemical elements, chemical formula.
1.1.6. Write down the signs of the chemical elements: sodium, iron, copper, iodine, fluorine, zinc It turns out that the atoms of these chemical elements are contained in fish, which is necessary as often as possible to eat. Find the material about the importance of these chemical elements for the human body.
1.1.7. Write down the names of the proposed chemical elements, a) from the first letters of the Russian names, you can learn the names of the product that contains a large amount of calcium, necessary for the growth and formation of bones and teeth Ti, H, Sn, Rb, Os, Hf b) from the first letters of the Russian names you will recognize the fruit, which is recommended for the prevention of aggressive manifestations; but it is better not to eat on an empty stomach, as it contributes to the formation of gases. It turns out that frozen fruit tastes the same as vanilla ice cream, it is no less sweet, and calories and fat in it is much less. Ba, Ag, Na, N, Ni.
1.2. Preparation of chemical formulas
· 1.2.1. Write down the chemical formula of glucose, if it is known that its molecule consists of six carbon atoms, twelve hydrogen atoms, six oxygen atoms. Glucose is a nutrient that helps to neutralize and eliminate poisons from the body
· 1.2.2. Write down the formula of carbon monoxide consisting of a carbon atom and an oxygen atom. Carbon monoxide can interact with blood hemoglobin, and this prevents the transfer of oxygen to the tissues, causing human death.
· 1.2.3.Make molecular and graphical formulas of substances: potassium chloride magnesium sulphate натрия sodium orthophosphate the sum of the indices in the formula of the first substance will help you determine the color that gives a person activity and optimism, is a symbol of happiness, readiness for change the sum of the indices in the formula of the second substance indicates a color that relaxes and soothes. This is the color of constancy, patience, hope, the sum of the indices in the formula of the third substance will determine the color of activity, but also irritability…
· 1.2.4. Make molecular and graphic formulas of sodium orthophosphate. Indexes will help you identify foods that contain phosphorus, which is necessary for brain cells: 1-walnuts, almonds; 2-sausage; 3-fish; 4-peanuts; 5-sausages.
· 1.2.5. In medicine and in everyday life often use menthol, chemical name-2-isopropyl-5-methylcyclohexanol-1. Make molecular and structural formulas. Menthol is a good antiseptic. Air-steam inhalations with this substance are indispensable for diseases of the upper respiratory tract. Protection from the common cold is boromentol-ointment of one part menthol, 10 parts boric acid and 189 parts vaseline.
· 1.3. Determination of the formula of substances by physical and chemical properties.
· 1.3.1. A chemical compound that exists in nature in the form of liquid, solid and gas, it accounts for about two-thirds of the human body. It has medicinal properties, is a good hardening agent. Turns the white powder of sulphate of copper in blue. Write down the molecular and structural formulas
· 1.3.2. Crystals of a black-gray substance with a metallic luster at n. u. can immediately pass from a solid state into a gaseous one (sublimation or voz-race), its solution gives a bright blue compound with starch. Its compounds are found in many drugs, 5% alcohol solution is used for disinfection, which leads to diseases such as "goiter" and "cretinism". Write down the molecular and structural formulas, explain the formation of a chemical bond in the molecule of the substance.
2. Calculations by the formula
2.1.12. Calculate how much substance is: a) water weighing 162 grams, b) water weighing 180 grams. The answers will tell you how many hours of sleep you need middle school student, to fully relax.
2.1.13. How much water will your body get if you drink its daily norm-138.9 mol?
2.1.14. Animals of arid countries use metabolic water, which is formed during biochemical reactions in the body. From fat mass of 100g. formed by-water amount of substance 6 mol. Calculate the mass of water formed from fat.
2.1.15. The villi of the human small intestine can suck 2 liters of water in 1 hour. How many water molecules can one villi suck in 1 minute, if the small intestine contains about 1 million villi?
2.1.16. Will drinking water be harmful to health if it is found in: a) 3,3 • 1 0 ' 6 mol / l iron ions (II); b) 1,7 • 1 0'7 mol/l of ions of Nickel (II); C) 1,9 • 10'7mol/l chromium ions (W)?
Sanitary standards allow the presence in drinking water of iron ions (II) in the amount of 0.2 g/m3 norm; Nickel ions (II) – 0.1 g/m3, chromium ions (W) – 0.05 g/m3.
2.1.17. How many iron atoms are contained in the hemoglobin of the blood of the average person, if the mass of these atoms is 3 g.? Hemoglobin acts as a vehicle for the transport of oxygen to the cells of the body.
2.1.18. To determine the amount of substance of copper contains 31• 1023 atoms. The answer will tell you the body's daily need for copper in milligrams. Copper is involved in the synthesis of hemoglobin and
2.1.19. In blood plasma, the ratio of the number of moles of sodium, potassium and calcium ions is strictly constant and is 25 : 1: 0.5 (this is the most important indicator of health, its change signals a disease). How many moles of these ions are contained in the blood of a healthy person, if the mass of sodium ions in it is 10g.
2.1.20. Clinical analysis of human blood shows that 100 ml of it contains 180 mg of potassium and 6.5 mg of calcium. How many atoms of potassium and calcium are contained in the blood of an adult, if the average volume is 5 liters? .
2.1.21. The human body contains a total of about 25 mg of iodine (as part of various compounds), and half of the total mass of iodine is in the thyroid gland. Calculate how many iodine atoms are: a) in the thyroid gland; b) in the human body as a whole?
2.1.22. The composition of the human body includes an average weight of 65% oxygen, 18% carbon, 10% hydrogen, 0.15% sodium, 0.15%chlorine. What atoms are more in the human body?
2.1.23. Calculate the number of molecules contained in ethyl alcohol weighing 69 g, as well as the mass of one molecule of alcohol. Ethyl alcohol is one of the psychoactive substances. At a concentration of 0.3 g/l, alcohol already causes a number of physiological and mental shifts. This concentration is retained in the body for 2 hours with the consumption of only 0.5 liters of beer.
2.1.24. What amount of substance is carbon dioxide, occupying a volume of 134.4 liters? Having calculated, you will learn how many minutes of life one smoked cigarette will score.
2.1.25. What amount of substance is ammonia, occupying a volume of 47 liters? Calculating, you will learn how many mg of strong nicotine poison co-holds one cigarette. The lethal dose of this poison is 40 mg, when Smoking 2/3 of the smoke gets into the air, so the smoke of the smoker is dangerous for others
2.1.26. Carbon monoxide (II), or carbon monoxide, is a dangerous pollutant in the atmosphere. Connecting with blood hemoglobin, it prevents the transfer of oxygen, causes diseases of the cardiovascular system, reduces the activity of the brain. Due to incomplete burning of topsoil on the Earth, 5 • 108 tons of this substance are formed annually. Determine what volume (at n. u.) will take carbon monoxide forming on the Earth for the specified reason.
2.1.27. Along with carbon dioxide, a person exhales carbon monoxide, about 1.6 ml (n. u.) for 1 hour. For what time will be reached the maximum permissible concentration of carbon monoxide, equal to 0.001 g / m3, if a person is in an isolated room with a volume of 6 m3.?
2.1.28. The daily requirement for vitamin D is 0.01 mg. Determine whether the norm of vitamin d intake will be met if you take 5 drops of 0.01% solution in oil once a day. The volume of one drop is 0.04 ml, the density of the solution is 0.92 g / ml
2.1.29. One drop of blood contains about 250 million red blood cells. Each red blood cell contains approximately 2.9 * 10-8 mg of hemoglobin. The molar mass of hemoglobin is about 67000 g/mol. Each hemoglobin molecule contains 4 iron atoms. How much iron can one drop of blood contain? How many oxygen molecules does 1 hemoglobin molecule attach?
2.2 Concepts: mass fraction of chemical elements, the establishment of a chemical formula for the mass fraction of chemical elements
2.2.1. Calculate the mass fraction of chemical elements (in%) in the following compounds: a) sodium sulfate; b) Epsom salt-seven-water magnesium sulfate; by the numbers corresponding to the correct answers you will learn: a) the most common external signs of Smoking 13-mobility; 32-destruction of teeth; 23-bad breath; 29-hair loss; 6-drowsiness; 45-gray complexion. b) what toxic substances enter the body together with tobacco smoke 13-mercury, arsenic; 7,3-calcium, chlorine; 9,8-ammonia, carbon monoxide; 23-formaldehyde, toluene; 12-glucose, fats; 5,7-acetone, svi-nets. Released harmful substances have a negative impact on those who are close to smokers.
2.2.2. By calculating the mass fraction of chemical elements in the molecule of ethyl alcohol, you will learn what diseases arise as a result of the evil use of alcohol. The answers of the problem correspond to the figures of the problems: 0.2-flu, runny nose; 0, 52-peptic ulcer, enuresis; 0.13-weakly-umie, hallucinations; 0.48-flat feet; 0.35-impotence In monatomic alcohols, with an increase in the number of carbon atoms in the molecule, their hemolytic effect increases (hemolysis - destruction of red blood cells with the release of hemoglobin into the external environment). Alcohols-hemolytic poisons (such as snake poisons)
2.2.3. The molecular formula of uric acid, which in case of improper metabolism is a dangerous product, as its salts are deposited in the joints, bones, brain, blood, leading to various diseases - C5H4N4O3, Calculate the mass fraction of chemical elements in its molecule.
2.2.4. Determine the formula of substances in which the mass fraction of the elements are: a) calcium 40%, carbon 12%, oxygen 48%, b) silicon 46.7 %, oxygen 53.3% C) calcium 38.7%, phosphorus 20%, oxygen 41.3% g) calcium 17%, hydrogen 1.7%, phosphorus 26.5%, oxygen 54.7% These substances are used in toothpastes as abrasive materials, which provide a cleansing and polishing effect. It is necessary to brush your teeth in the morning and in the evening to prevent dental deposits that cause caries and periodontal disease-the destruction of teeth.
2.2.5. Traces of a substance with an anti-toxic and narcotic effect were found in drinking water. During qualitative and quantitative analysis, it was found that this phenol derivative and the mass fractions of chemical elements in it are as follows: 55 % (C), 4.0 % (H), 14% (O), 27 % (C1). Set the molecular formula of the substance. Indicate possible causes of release of this substance into the environment.egative impact on those who are close to smokers.
2.2.6. The content of chemical elements in the human body (by weight %) O-63% C-21% H-10% N-3% Ca-2% P-1% K-0.27% S-0,16 % Na-0,10 % Cl-0.08 % metals accounted for 3% of the mass of a person Calculate the mass of each chemical element in your body.
2.2.7. Calculate the mass fraction of chemical elements in the molecule of alkane, the hydrogen density of which is 15, and you will find out how many percent of the responsibility for the preservation of dental health depends on the person himself (a large figure), and how many percent
2.2.8. The mass fraction of inorganic substances in human bones is 22%. The mass fraction of calcium hydrophosphate is 85%, and the mass fraction of bones in the human body is 16-18%. Determine the mass of calcium hydrophosphate and calcium element in the human body weighing 70 kg.
2.2.9. The mass fraction of human bones is 20% of the total body mass. The proportion of calcium phosphate, which is part of the bones, accounts for 20% of the bone mass. Knowing your weight, calculate how many kilos of calcium phosphate you have in your body. How many kilograms of calcium does it contain?
2.2.10. The mass fraction of iodine in the thyroid gland is 0.12%. Thyroid mass 40g. Determine the mass of iodine, which is contained in the thyroid gland.
2.2.11. Calculate the mass of water in your body if it is known that the human body contains 68% of this substance. Indicate in which organs more water is concentrated.are guaranteed by dentists.
2.2.12. Bromine content in the brain is 15-30 mg per 100 g of tissue. The mass of the adult brain is about 900-1200 g. What is the mass of bromine contained in the brain?
2.2.13 how much sodium monophosphate NaF * NaPO3 is contained in a tube of toothpaste weighing 100g, if the package contains information that the content of active fluoride is 0.15%?
2.2.14 Dentists recommend to use 1.5 g of active fluoride annually for the prevention of caries. How many tubes of toothpaste should be used per year to ensure this rate, if it is known that a tube weighing 100g contains 0.15 % of active fluoride?
2.2.15 Set the formula of the crystallohydrate, the mass fraction of aluminum sulfate in which is 0, 5135. Aluminum sulfate is not a medicinal product, but is used for water purification. When adding a small amount of aluminum sulfate to the water, a colloidal solution of aluminum hydroxide is initially obtained, which then coagulates, giving a voluminous gelatinous precipitate, capturing particles and bacteria suspended in the water during its formation, and drags them to the bottom of the settling tank.
2.2.16 talc - magnesium silicate is often used for the manufacture of tablets and pastes. It is also used as a powder. Set its simplest formula if it is known to contain 31.75 % magnesium oxide, 63.49 % silicon oxide and 4.76% water
2.2.17 Lack of movement leads to disruption of phosphorus-calcium metabolism, which ultimately leads to urolithiasis. Stones, which are formed in the urinary tract, have a different composition. Determine the simplest possible formula of renal cells, if it is known that their composition in addition to 6 mol of water, contains MD-17.52%, N-10.21%, H-2.92, P-22.63%, O-46.72%
2.2.18. Bitter, or English, salt (magnesium sulfate crystallohydrate) was first isolated from the water of a mineral spring in Epsom-a suburb of London. This salt is used in medicine for diseases of the nervous system, to reduce blood pressure, as well as as a laxative. Make the formula of the English formula, if the mass fraction of chemical elements in it are: 9.86 % (Mg), 13.01 % (S), 71.40 % (O), 5.73 % (H). Formula of the English formula, if the mass fraction of chemical elements.
2.2.19. A vital task is to maintain the balance of molecular oxygen in the air. The boiler house burns 2 tons of coal per year. Composition of coal: C-84%, H-5%, H2O-5%, S -3.5% by weight. Given that 1 ha of birch forest per year gives 725 kg of acid, calculate from what area of the birch forest will fill-SIA spent on burning coal during the day acid?
3. Solutions
3.1. Solution preparation
3.1.1. During training in GO and emergency at school students are advised in the case of: a) air pollution with chlorine can be protected by gauze bandages moistened with 5% solution of baking soda and climb to the top floor of the school building; b) pollution of atmosphere by ammonia to put on the gauze dressings moistened with 3% solution of acetic acid and to settle down on the first floors. How to prepare such solutions weighing 500g.? Explain these phenomena from the chemical point of view
3.1.2. When providing first aid for burns with white phosphorus, a 5% solution of soda is used. How much soda should be taken to prepare 600 g of RAS-tvor?
3.1.3. Potassium permanganate-KMnO4 is used in medicine as a 5% solution to lubricate burned areas and as a hemostatic agent. Determine the mass of potassium permanganate and water required for the preparation of 50 g of this solution Weaker solutions are used to rinse the mouth and throat as a disinfectant due to its high oxidizing ability.
3.1.4. In medicine, an alcoholic solution of trinitroglycerin, the mass fraction of which is 1%, is used as a cardiac drug (for angina). Determine the mass of trinitroglycerin required for the preparation of such a solution weighing 15 kg.
3.1.5. Solution, the mass fraction of phenol in which 0.5% is widely used for disinfection. Determine the mass of water and phenol needed to prepare such a solution weighing 1.5 kg.
3.1.6. A solution of sodium chloride 0.85 - 0.9% is called a physiological solution and is used for intravenous infusions with large blood losses. Determine the mass of water and salt required to prepare 5 kg of this solution
3.1.7. Powder "Rehydron" is used for dehydration. One dose of the powder contains 3.5 g of sodium chloride, 2.5 g of potassium chloride, 2.9 g of sodium citrate and 10 g of glucose. Before use, the dose is dissolved in 1 liter of water. Determine the mass fraction of all components of the powder "Re-Hydron" in the resulting solution.
3.1.8. Why is it recommended to take if gasoline gets into the stomach inside vegetable oil? Determine the mass fraction ( % ) of carbon monoxide in the resulting solution, if after swallowing 2G of Ben-zine, the victim drank 50g of vegetable oil.
3.1.9. Formalin contains formaldehyde (mass fraction 0.37) is used for disinfection. Calculate how many moles of formaldehyde will be required to prepare 3 kg of solution. What is the molar concentration that corresponds to a mass fraction of formaldehyde in the solution?
3.1.10. Acz-a means from cough. One dose of ADC weighing 3 g contains 100 mg of acetylcysteine and 2.9 g of sucrose. Before use, the ADC is dissolved in 100 ml of water. Determine the molar concentration of sucrose in the irradiated solution, if the density of the solution is 1.01 g / ml. mouth and throat as a disinfectant due to its high oxidizing ability.
3.1.11. Determine the mass of fluorine in the blood contained in the human body weighing 80 kg, if the blood volume of 10 ml contains fluorine weighing 0.01-0.07 mg, and the mass fraction of blood in the human body is 8% (blood density 1.05 g / ml).
3.1.12. What is the mass of perhydrol (30% solution of hydrogen peroxide H2O2) and water required for the preparation of 100g 3% solution of hydrogen peroxide, used in medicine for the treatment of wounds and abrasions. Explain this process from a chemical point of view.
3.1.13. Glucose in medicine is often used in the form of solutions of different concentrations, which serve as a source of fluid and nutrient material, as well as contribute to the neutralization and excretion of poisons from the body. Calculate in what mass of glucose solution with a mass fraction of 5% should be dissolved 120 g. it to obtain a solution with a mass fraction of glucose 8%.
3.1.14. The pharmacist was instructed to prepare eye drops, which are an aqueous solution of zinc sulfate and boric acid (the mass fraction of zinc sulfate-0.25 %, boric acid-2 %). Determine the weight of zinc sulfate and boric acid, which are necessary for the pharmacist to prepare drops, if distilled water he took 200 ml.
3.1.15. For wound treatment, a 5% alcohol solution of iodine containing 2% potassium iodide is used. 95% ethanol solution is used for preparation of such preparation. Determine the mass fraction of water in an alcohol solution of iodine.
3.1.16. In peptic ulcer patients are prescribed to drink 0.05 % solution of silver nitrate. The daily dose of silver nitrate is 0.1 g. for how many days will the patient have 2l of 0.05% silver nitrate solution? The density of this solution is considered equal to the density of water.
3.1.17. With reduced acidity of gastric juice, patients are prescribed diluted hydrochloric acid, in which the mass fraction of hydrogen chloride is 8.2 % (p = 1.04 g / ml). In the pharmacy, it is prepared from 37 % hydrochloric acid (p = 1.19 g / ml). Determine the amount of dilute acid that can be prepared from 20 ml of 37 % hydrochloric acid.
3.1.18. The patient received intravenously as an antiallergic agent 10 ml of 30% sodium thiosulfate solution (p = 1.2 g / ml). How many sodium ions got into his body?
3.1.19. Six-water calcium chloride crystallohydrate, coming to pharmacies, is not used for the preparation of drugs, as it is gig-roskopichen and has a variable composition, which can lead to an inaccurate dosage. A 50% solution of calcium chloride is prepared from the crystallohydrate, which is then used for the preparation of drugs. Determine the weight of the crystallohydrate that will be required to prepare a 50 % solution, if the pharmacist has 100 ml of distilled water. Determine the mass of water and 50% solution of calcium chloride required to prepare 100 g of 10 % solution of this substance.
3.1.20. In 184 g of water dissolved 16 g of copper sulfate. What is the mass fraction of copper (II) sulfate in the resulting solution? The solution of this concentration in medicine is used for skin burns with phosphorus, 25% solution of copper sulfate (II) is used as an astringent and anti-septic agent-eye drops;
3.1.21. Bitter, or English, salt (magnesium sulfate crystallohydrate MgS04 • 7H20) is used in medicine as a laxative, which is due to the almost complete impenetrability of the intestinal walls for Mg2 + ions. Calculate how much crystal salt and water you need to take to prepare 400g of magnesium sulfate solution with a mass fraction of 8%.
3.1.22. Determine the mass fraction of zinc sulfate in the solution obtained by dissolving 25 g of zinc sulfate heptahydrate-ZnSO4· 7 H2O in 100 g of 4% zinc sulfate solution. Zinc sulfate is included in eye drops as an anti-inflammatory agent.
3.1.23. When evaporating a solution of sodium sulfate salt is released in the form of crystallohydrate Na2SO4·10 H2O. Determine the mass of the crystal-loghydrate, which can be obtained from 200 ml of solution with a mass fraction of sodium sulfate 0.15, the density of which is 1.14 g / ml. Na2SO4·10 H2O-Glauber's salt, transparent crystals of bitter-salty taste, is used as a laxative, and as an antidote for poisoning with barium and lead salts, with which it gives non-soluble salts.
3.1.24. What amount of anhydrous sodium sulfate corresponds to the daily dose of the medicine, 200 ml of which contains 30 g of sodium sulfate crystallohydrate (Na2S04-10H2O). The daily dose of the medicine is 30 ml.
3.1.25. In a saturated solution of iron (III) chloride, another 5g of salt was dissolved when heated, and then the solution was cooled to the initial temperature. Determine the mass of the precipitated crystals of iron (III) chloride hexahydrate-Fe Cl3· 6 H2O, if the mass fraction of anhydrous salt in the aqueous solution is 0.5. Iron (III) chloride solution is used in medicine as a disinfectant and blood-stopping agent.
3.1.26. What do you know about saturated solutions? In many river fish, the caviar is poisonous, so the abdominal cavity of any fish after gutting should be washed with a saturated solution of table salt. Explain why just pure water is not used.
3.1.27. The daily requirement for vitamin D is 0.01 mg. Determine whether the norm of vitamin d intake will be met if 5 drops of 0.01% solution in oil are taken once a day. The volume of one drop is 0.04 ml, the density of the solution is 0.92 g / ml
3.2. Solubility
3.2.1. Calculate the mass of water and anhydrous copper sulfate required for the preparation of a saturated solution from which, when cooled from 100 to 20 ° C, 100 g of copper sulfate Ciso4·5 H2O falls out, if the anhydrous salt content at 20 ° C and 100 ° C is equal to 21 g and 75 g. per 100 g of water. Find the material on the use of this substance as a drug and the role of copper ions in the human body.
3.2.2. The solubility of lead nitrate is at 0oC-28 g, and at 100oC-125 g. Calculate how much salt will be released from 200 g of the solution, saturated at 100oC and cooled to 0oC. Lead compounds are poisonous, they accumulate in the body and can cause poisoning even in acute
3.2.3. The solubility of copper sulfate at 0 ° C 15 g per 100 g of water. How many grams of saturated copper sulfate solution can be prepared from 100 g of ciso4·5 H2O crystallohydrate? Copper sulfate solutions (0.25 -0.5%) are used in the treatment of acute inflammatory skin diseases.form.
3.2.4. The solubility of calcium chloride at 00C and 210 C is equal to 60 and 75 g of anhydrous salt per 100 g of water. How much calcium chloride hexahydrate is produced by cooling 700 g of saturated solution from 210 to 00? Calcium chloride solution is used as an anti-inflammatory, decongestant, antiallergic agent, as it reduces the permeability of the capillary walls, as well as has a calming effect in the treatment of neuroses, bronchial asthma, tuberculosis
3.3 solution Medium
3.3.1. Purgen (phenolphthalein) is used as a laxative. What kind of color will gain a snowstorm in solutions
§ ammonia
§ baking soda
§ ethyl alcohol?
Answer motivate. Experience the effect of Purgen on office glue at home. How to explain the observed phenomenon?
3.3.2. Gastric juice is a colorless liquid that has an acidic re-action of the medium due to the presence of hydrochloric acid HC1, which is among the strong acids. Calculate the pH of the jelly juice, if the mass fraction of HC1 in it is 0.5 %. The density of gastric juice is almost equal to the density of water.
3.3.3. In the blood plasma as a result of metabolism, the following compounds can accumulate in the form of ions: sodium bicarbonate, ammonium dihydrophosphate, ammonium hydroxide and ammonium hydrophosphate. How is the acidic and alkaline environment eliminated in this case? Which components will interact (in pairs) to neutralize the environment? Write the equations of possible reactions. home. How to explain the observed phenomenon?
3.3.4. Aspirin (acetylsalicylic acid-CH3SOOS6H4SOON-ester formed by acetic and salicylic acids) is sometimes used as a preservative additive. Is this application possible? Prove what chemical reactions can occur in the process of preserving products, and how this will affect the chemical properties of the drug.
3.3.5. Many microbes die in an alkaline environment. Think, solutions of what substances contained in the kitchen or in the medicine Cabinet, you could use to create an alkaline environment for inflammatory diseases of the oral cavity, nasopharynx (runny nose, stomatitis, etc.)
3.3.6. Proteins include organic acids such as glycine H2N-CH2-Coon, aspartic noos-CH2-CH(NH2) - Coon, Li-Zin H2N - (CH2)4-CH (NH2) - Coon. What is the color of the indicator will have the solutions of these acids explain.
4. Problems on equations of chemical reactions
Did you know that…
• about 2000 chemical reactions take place simultaneously in one cell of human and animal organism
• every second in our brain there are about 100 thousand chemical reactions
4.1. Classification of organic and inorganic substances, their chemical properties
Oxides:
4.1.1. Write down the equations of chemical reactions:
(a) phosphorus oxidation (V);
b) the combustion of ammonia in the absence of a catalyst;
C) oxidation of ammonia in the presence of a platinum catalyst;
d) decomposition of iron nitrate (III);
Equalize, add up the coefficients in the equations of chemical reactions and you will learn:
a) at what time does a person have the highest efficiency;
b) at what time does a person have the greatest fatigue;
in) in what time have human evening rise?;
d) when it is necessary to stop any activity;
The use of the acquired knowledge about biological rhythms in the composition of the day regimen will allow to achieve maximum efficiency and increase the body's resistance to fatigue.
4.1.2. Zinc oxide is included in the zinc ointment used for skin diseases, as it has astringent, drying and disinfecting properties, and a bath with several tablespoons of magnesium oxide relieves tension of the nervous and muscular system. Which of the proposed substances may interact with zinc oxide and magnesium oxide: CO2; Na2O; KOH; H2SO4; H2O? Write down the equations of possible chemical reactions.
4.1.3. N2O - "laughing gas", mixed with oxygen is used as an anesthetic. As you understand, this oxide is non-forming. Which of the proposed names are possible for this oxide: nitrogen monoxide, diazot oxide, nitric oxide (I), nitrous oxide, nitrogen dioxide?
4.1.4. Arrange the coefficients in the equation of the chemical reaction between aluminum and oxygen, from the letters corresponding to the coefficients, make a word, and you will learn what enemy, along with Smoking, has a destructive effect on the body. 1-a; 3-y; 2-m; 4-W; 5-g; 6--f.
Bases
4.1.5. Aluminum hydroxide is a part of the adsorbing and enveloping agent used in gastric ulcer and gastritis. From the proposed reagents (KOH; AlCl3; H2SO4; KNO3) you-take the reagents necessary to obtain aluminum hydroxide, as well as reagents that characterize its chemical properties. Conduct possible chemical reactions, write down the equations of chemical reactions in molecular and ionic form.
4.1.6. .For a substance having the molecular formula CA (OH) 2, there are many names. Select from the list of suggested names those that correspond to it. Explain the essence of the name: quicklime, calcium hydroxide, slaked lime, fluff, lime mo-Loco, lime water. This substance in the form of lime water is used externally and internally as an anti-inflammatory, astringent, de-infecting agent. Explain the formation of chemical bonds when the gas dissolves in water.
4.1.7. Colorless gas with a pungent smell, when burning, emits water and nitrogen. 10 % aqueous solution is used as a drug for fainting: the gas released from the solution irritates the nerve endings of the upper respiratory tract and reflexively excites the Central nervous system — a person regains consciousness. Write down the molecular formula of the substance. Explain the formation of chemical bonds when the gas d4.1.8.. In case of heartburn and stomach pain, Maalox is used, containing in 100 ml of suspension 3.49 g of aluminum hydroxide and 3.99 g of magnesium hydroxide. How many moles of hydroxides enter the human body when taking -1 tablespoon (15 ml) of the drug?
4.1.9.Among the substances of the formula, which NH3* H2O; H2S; BA (OH)2; C6H5OH; NH2CN2COH, select substances with basic properties. Write down the equations of possible chemical reactions with hydrochloric acid. How are these substances related to human health?
Acids
4.1.10. Make equations of chemical reactions, and by the number of substances involved in the reaction you can determine the products that a) stimulate metabolism in the brain and thus facilitate the learning process: sodium carbonate and hydrochloric acid:
1-carrots; 2-pineapple; 3-peas; 4-bread
b) facilitate the perception of information and restore strength: aluminum with sulfuric acid solution: 1-tomato; 2-lemon; 3-grapefruit; 4-sausages
C) neutralize negative emotions: § iron with chlorine: 1-cucumber; 2-banana; 3-strawberry; 4-Apple
4.1.11. The composition of gastric juice contains hydrochloric acid. Excess of it (increased acidity) causes heartburn in the body. If hydrochloric acid is lower than normal, then a person has low acidity. When "heartburn" at home often take drinking water. Write down the equations of the chemical reaction. Is it possible with the help of baking soda to get rid of "heartburn" forever? Why?
4.1.12.. When poisoning doctors advise to wash the stomach, using a large amount of water. But causing vomiting and washing the stomach with water is contraindicated in sulfuric acid poisoning. Why? Explain the safety rules for the preparation of sulfuric acid
Sols
4.1.13. Sodium sulfate (Glauber's salt-Na2SO4. 10 H2O) - transparent crystals of bitter-salty taste, used as a laxative, and as an antidote for poisoning with barium salts and lead, which gives insoluble salts. Write down the equations of possible chemical reactions in molecular and ionic form.
4.1.14. Barium sulfate is used in medicine as a radiopaque medium, but it is unacceptable admixture of barium carbonate. Known cases of poisoning, if barium sulfate was present admixture of barium carbonate. Explain the cause of poisoning and how to avoid it?
4.1.15. When cauterizing the throat, lapis (silver nitrate) was used, but when it gets inside, it turns out a terrible burn of the throat, stomach, intestines with excruciating pain. Before the arrival of the doctor, the patient is given a solution of table salt. Why? Write down the equation of the chemical reaction.
4.1.16. When poisoning with acids, it is necessary to use alkalis. But alkalis act on the body devastatingly. It is often suggested to use substances such as: a) chalk, b) magnesia, C) lime milk. What are these substances? Explain the chemical nature of the processes taking place.
4.1.17. When poisoning with heavy metals, sodium thiosulfate is often used as an antidote. Write down the equation of chemical reaction between sodium thiosulfate and mercury (II) chloride solution. Explain the essence of its use.
4.1.18. With improper care of the oral cavity, the teeth become very sensitive to hot and cold food, but these sensations pass if you brush your teeth twice a day with a paste containing fluoride. How to explain this fact from a chemical point of view?
Hydrocarbons
4.1.19. Iodoform CH3I has an antiseptic effect, because in contact with the wounded surface of the tissue is oxidized by the oxidative enzyme catalase of blood with the release of iodine, hydrogen oxides and water. Write down the equation of the chemical reaction of oxidation of iodoform with atomic oxygen.
4.1.20. Discuss the issue of obtaining chloroethane in several ways. Write the equations of a chemical reaction. Ethyl chloride evaporates rapidly when liquefied gas is applied to the skin, it is strongly cooled, and is used in medicine for local anesthesia
4.1.21. Chloroform CHCl3 has a strong narcotic and anesthetic effect, is used in medicine. Is it possible to obtain this substance from ethane, acetic acid. Write down the equations of possible chemical reactions.
4.1.24. Methane is often the culprit in mine explosions. Write down the equation of the chemical reaction. Explain under what conditions this can happen, given the chemical inertness of alkanes.
Oxygenated
4.1.25. To preserve the structure of toothpastes, reduce its freezing point, plasticity and increase the stability of the foam formed when brushing the teeth, polyatomic alcohols are introduced into the paste: sorbitol, glycerin, propylene glycol. Write down the molecular and structural formulas of these compounds, name them by international nomenclature.
4.1.26. If sodium bicarbonate is present in the composition of the paste, it neutralizes lactic acid and increases the effectiveness of the Pro-lactic action of the paste against caries. Write down the equation of the chemical reaction taking place.
4.1.27. A valuable sugar substitute for people with diabetes is sorbitol, which can be obtained from glucose in an acidic environment (HCl) when exposed to zinc. Write the equations of the corresponding chemical reactions.
4.1.28. Xylitol, a five-atom alcohol with a chilling sweet taste-a substitute for sugar for diabetics. Make the reaction equation for obtaining xylitol from ribose.
4.1.29. The composition of butter, which is the most important human food, unlike other fats, includes glycerol ether with butyric acid. Write the structural formula of such an ether with oleic, palmitic and butyric acids.
4.1.30. In the composition of sparkling grape wines found fragile ether of carbonic acid and ethanol. Write the reaction equations for the formation of such an ether. Call it.
4.1.31. The soaked rice can be stored for a long time without drying, having processed it previously with a solution of propionic acid with a mass dose of 0.03. Get called a preservative, proceeding of monochloro-pan.
4.1.32. Make the structural formula of the drug aspirin is acetylsalicylic acid if it is known to be produced by reacting salicylic acid (NO-C6H4-Coon)with acetic anhydride (CH3SO) 2O and the by-product of re – action is acetic acid.
4.1.33. Lactic acid (CH3SNONSOON) is contained in muscle tissues, it accumulates in the production of all kinds of lactic acid products, during salting, silage. As a result of what chemical reactions it can be formed? Write down the equations.
4.1.34. Using the following statements, make your own tasks:
* in the homologous series, the strength of narcotic action and toxicity of substances increases with the increase in the number of carbon atoms in the molecule.
* branching of carbon chains weakens narcotic and toxic effects, when the chains are closed, the toxicity of substances increases.
* the presence of multiple bonds increases the chemical activity of organic compounds, which in turn not only causes an increase in narcotic and toxic effects, but can change the nature of the impact of the substance, for example, such a compound has an irritant effect.
Nitrogen
4.1.34. Dimethylamine is used in the synthesis of the drug AMI-nazine. With which of the proposed substances dimethylamine will act: H2; NaOH; HCl; H2O. . Write down the equations of chemical reactions.
4.1.36. Aminoacetic acid (glycine) - sold in pharmacies as a drug. It plays an important role in the neutralization of toxic substances that have entered the body. Prove the dual function of this acid. Write the equations of chemical reactions.
4.1.37. The acid serine NH2 CH NH2 COH is a part of proteins. Name it by systematic nomenclature. Make a forecast of its chemical properties.
4.1.38. When replaced in the benzene nuclei of the two meta carbon atoms on the nitrogen atoms, it turns heterocodeine – pyrimidine. Write down its structural formula. Pyrimidine ring is a part of many biologically active substances (nucleic acids, some vitamins, drugs). Oxygen derivatives of pyrimidine-uracil, thymine and cytosine are part of nucleic acids (RNA and DNA). Write down the structural formulas: Uracil – 2.4 dioxopyrimidine Thymine-2,4 dioxy-5-methyl pyrimidine Cytosine-2-oxy-4-aminopyrimidine
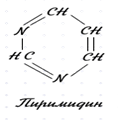 (pyrimidine)
(pyrimidine)
Calculations according to the equations of chemical reactions
4. 2. Calculations for a given mass of substances
4.2.1. One of the solutions, which not only quenches thirst well, but also reduces the need for drinking for a while, consists of citric acid, calcium chloride and potassium chloride. You can pre-prepare a mixture of solids: sugar 5 g, citric acid 2 g and salts of 0.025 g per 1 liter of water. Citric acid promotes the removal of Ca2 + ions from the body, and calcium chloride will maintain the salt balance of the body. What amount of calcium chloride will be obtained by the action of excess acid per 200 g of calcium carbonate? What mass of water and carbon dioxide will be released?
4.2.2. When a person suffers from heartburn, he uses baking soda. Even better, if he uses drugs containing magnesium hydroxide in his body. Calculate the mass of magnesium hydroxide required to neutralize 1.2 g of hydrochloric acid, which caused the increased acidity of gastric juice. 4.2.3. Iron – the most important component of the human body and animals. For the treatment of anemia caused by iron deficiency in the body, and to stimulate the work of hematopoietic organs, restored iron is used. Calculate the mass of iron that can be reduced by aluminum from 1.5 moll of iron (III) oxide.
4.2.4. Spilled mercury in the laboratory can be collected using paper or metal plate coated with copper. Then the mercury balls should be poured into the fume hood with concentrated nitric acid, and the place where the mercury was covered with sulfur. Make the reaction equations. What products, and in what quantity are formed when processing 2 g of mercury with concentrated nitric acid?
4.3.Calculations related to the volume of substances
4.3.1. When breathing, a person consumes for 1 hour 56 liters of oxygen (n. u.) What mass of glucose can be oxidized in the body by this amount of hydrogen?
4.3.2. Chloroform is used for anesthesia. Determine the mass of methane and chlorine required to produce 500 ml chloroform if its density is 1.48 g / ml.
4.3.3. For local anesthesia during surgery, it is necessary to prepare gaseous ethyl chloride with a volume of 5.6 liters. What volume of ethylene and hydrogen chloride will be required for this?
4.3.4. Sodium peroxide is used in submarines to absorb carbon dioxide and replenish oxygen in the air. What volume of gas can react with 78 kg of sodium peroxide, and what volume of oxygen is formed?
4.3.5. In laboratory spirit lamps, ethyl alcohol burns with the release of carbon dioxide and water. Calculate the amount of carbon dioxide that has accumulated in the chemical room with a capacity of 228 m3, if each of the 18 tables during the work of students burns 2.3 g of alcohol. Calculate the volume fraction of carbon dioxide and explain whether it will affect the well-being of students, given that the volume fraction of carbon dioxide in the air is 0.03%, and if its content is higher than 4% there is irritation of the respiratory tract, there is noise in the ears headache.
4.3.6. Calculate to what value the oxygen content will decrease from the usual (21% by volume) in the air of a room with a volume of 45 m3 in 10 hours due to the respiration of plants weighing 4 kg and the average intensity of respiration of 12 ml of oxygen per 1 g per day (normal conditions).
4.3.7. Chlorine is a very poisonous gas. For its absorption in the first gas masks used sodium thiosulfate. The reaction takes place according to the equation: Na2S2O3 + 4Cl2 + 5H2O = 2H2SO4 + 2NaCl + 6HCl. How much chlorine can absorb 4.74 g of sodium thio sulfate? What volume of concentrated hydrochloric acid solution with a mass fraction of HCl 35% (p = 1.174 g / cm3 at 20ºC) should be spent on its preparation?
4.4. Calculations related to solutions
4.4.1. For x-ray examination of the stomach, the patient is given a drink suspension of barium sulfate. Why use sulfate and not chloride? How can barium sulfate be obtained using sulfuric acid? What weight of 49% sulfuric acid should be taken to obtain 100 g of barium sulfate?
4.4.2. Iodine tincture contains 5% iodine and 2% potassium iodide (by weight). Su-the exact need of the body for iodine 200 mcg (1 mcg = 10-6 g). Calculate the mass of iodine tincture, which has this portion of iodine. How to prove that iodine tincture contains potassium iodide? Write down the equation of the chemical reaction, determine the mass of the precipitate released by the action of the reagent on a solution containing 2 g of potassium iodide.
4.4.3. The simplest way to remove spilled mercury is to treat the contaminated site with iodine tincture. What mass of iodine tincture containing 5% iodine should be used to destroy 2 g of mercury?
4.4.4. In the pharmaceutical industry, when ethyl alcohol is degridated at 1400 C in the presence of concentrated sulfuric acid, diethyl ether is obtained. What is the mass of ethanol with a mass fraction of 96% needed to produce diethyl ether weighing 148 kg?
4.4.5. The daily requirement for fats for a person is by weight 106-163 g depending on the profession. What is the mass of glycerol and stearic acid formed by enzymatic hydrolysis of the monthly rate of tristearin?
4.4.6. For stomach fluoroscopy, a suspension of barium sulfate in water is used. Barium sulfate is obtained from the mineral viterite, consisting mainly of barium carbonate. Calculate the mass of 35 % hydrogen chloride solution, which will be required for the complete dissolution of 100 g of viterite containing 5 % non-carbonate impurities. Determine the mass of 20% sodium sulfate solution required for complete precipitation of barium ions as sulfate from the resulting barium chloride solution.
5. Oxidation-reduction reactions.
5.1. Write down the equations of chemical reactions and the number of electrons involved in the oxidation process, determine how the surrounding colors affect the human condition:
1. Oxidation of aluminum: red: 1-sadness, 2-calm, 3-activity and irritability, 4-concentration
2. Interaction of zinc with hydrochloric acid; yellow: 1-distraction, 2-activity and optimism, 3-passivity and weakness, 4-melancholy
3. Interaction of iron with chlorine: green: 1-excitement, 2-aggressiveness, 3-calm, 4-fatigue
4. Burning sulfur Gorenje: blue: 1-aggressiveness, 2-openness, 3-irritability, 4-tenderness
According to scientists, the color and interior of the room reflect the habits of a person, largely determining his mood and well-being. Choosing the color scheme of the room, it is necessary to take into account its illumination. Sufficient illumination relieves fatigue.
5.2. Sulfur and its compounds are drugs. Write - those at least six different reactions in which sulfur changes its oxidation state 5.3. The sulfur compound, which has only restorative properties, is used in medicine to treat rheumatism and skin diseases, but in a mixture with air it is poisonous and explosive. Sulfur compound, which has oxidizing properties, vigorously absorbs water from the air and is dangerous to human life. Write down the formulas of these compounds and the equations of redox chemical reactions.
5.4. In case of hydrogen sulfide poisoning, the victim is allowed to breathe diluted bleach, from which small amounts of chlorine are released. At a poisoning with bromine to give to breathe the ammonia fumes. Write down the equations of possible chemical reactions.
5.5 what substances in the home medicine Cabinet, as disinfectants, are used to produce oxygen. Write down the equations of chemical reactions and their conditions
5.6 in the home medicine Cabinet such substances as potassium permanganate, ammonia, ethyl alcohol are stored. Discuss the possibility of interaction between them. Write the equations of possible reactions, specify the conditions in which they occur. What do you know about their use as medicines?
5.7. Using substances from the home medicine Cabinet, the student tried to conduct chemical reactions. What substances it needs to take, and under what conditions to carry out chemical reactions, if it assumes to produce the following substances (all reaction products are specified without coefficients):
· MnO2 + СН3СНО + КОН + H2O
· MnO2 + N 2 + KOH + H2O
5.8. In iodine tincture the following transformations can occur: J2 + H2O = HJ + HJO (oxidizer); Alcohol + oxidizing agent → aldehyde → acid→ ester. Write the reaction equations and name the substances formed.
5.9 Glucose was subjected to complete oxidation with potassium permanganate and sulfuric acid. Write down the equation of the chemical reaction using the method of half-reactions (ion-electron balance). Explain how glucose is formed in living organisms (plants) and what its role is.
6. Calculations on excess substances.
6.1. With a lack of zinc in the human body, there is a slowdown in growth, a violation of the skin and hair. Calculate the weight of zinc sulfate, which is the annual physiological requirement of human organism, which is formed by the interaction of 0.14 g of zinc with sulfuric acid weighing 0.16 g.
6.2. Calculate the mass of baking soda (sodium bicarbonate) used in medicine for rinsing and inhalation, which can form when passing 2.64 g of carbon dioxide through a solution containing 2 g of sodium hydroxide.
6.3. Purified sulfur in combination with some organic additives (vaseline, peach oil) is used in medicine for the treatment of various skin diseases and as an antiseptic. Write down the equation of the Chi-nomic reactions combustion of sulfur in excess oxygen. Calculate the mass of sulfur oxide (VI) obtained during the combustion of 128 g of sulfur in 120 l of oxygen.
6.4. Ammonium chloride (ammonia) is used as a diuretic and expectorant NH4Cl What is the mass of ammonium chloride can be obtained by reacting 30g of ammonia with 75g of hydrogen chloride?
6.5. Phosgene (CO2) - a poisonous gas with a suffocating smell acts on mucous membranes and respiratory organs of humans and animals. In the First World War it was used as a combat poison. What volume of phosgene can be obtained by the interaction of 20L chlorine and 10l carbon monoxide? Write down the possible structural formula of phosgene.
7. Product yield.
7.1. Ammonia is a substance that is a product of the life of living beings. - find the material on the formation of ammonia in the human body;
- remember the industrial method of producing ammonia;
- it took 12 g of hydrogen to produce 64 g of ammonia. Calculate the mass fraction of the output of the product;
- what volume of ammonia can be obtained by reacting 20 liters of nitrogen with excess hydrogen, if the volume yield of the product is 80% of the theoretically possible?
7.2. It is known that glycerin is used in the production of wines, medicine, pharmaceutical industry. Calculate the mass of glycerol obtained by hydrolysis of 10 molе fat tristearide, if the practical yield of the product is 75% of the theoretically possible.
7.3. At the end of the shelf life guaranteed on the label, the fat may taste sour. This is a consequence of fat hydrolysis. Write down the equation of the chemical reaction in General. Calculate what mass of glycerol can be obtained if it took 540 kg of water for hydrolysis, and its yield is 80%.
7.4. From acetylene volume of 3.36 liters (n. u.) received 2.5 liters of benzene. The density of benzene is 0.88 g / l. Determine the mass fraction of the product yield. Benzene is physiologically very active, its vapors at high concentrations act on the Central nervous system, liquid benzene strongly irritates the skin.
7.5. Ammonium chloride and ammonium hydroxide are used in medicine and have the following names: ammonia and ammonia. Write down the molecular formulas and determine the correspondence of their names. What is the mass of ammonium chloride can be obtained from ammonia volume of 10l, if the mass fraction of the product yield is 80%?
7.6. When salicylic acid (NO-C6H4 - Coon) interacts with acetic anhydride (CH3SO)2O, acetylsalicylic acid, known in medicine as aspirin, is obtained. (CH3OS-O-S6N4-soon) and UC-susic acid. What weight of aspirin can be obtained from 690 kg of salicylic acid, if the mass fraction of the product yield is 75%?
7.7. Calculate what volume of 10% hydrogen chloride solution in water (density 1047 kg / m3) can be obtained from 58.5 kg of sodium chloride, if the yield of hydrogen chloride is 68% of the theoretical. Hydrochloric acid (hydrogen chloride solution) is formed in the stomach (0.2%).With an excess of it, there is "heartburn". Offer a quick way to get rid of heartburn at home.
7.8. The greenhouse effect is a gradual warming of the climate on the planet as a result of an increase in the concentration of greenhouse gases (CO2, CH4, O3 and others), which prevent the departure of long-wave radiation from the surface of the earth. One of the main sources of carbon dioxide is the burning of fossil fuels. What volume of carbon monoxide (IV) will be obtained in the gas generator from 1 ton of coal containing 92% carbon, if the loss in production will be equal to 15% (n. u)?
8. Calculations on mixtures of substances.
8.1. At the interaction of 7.8 g of a mixture of methanol and ethanol with sodium metal, 2.24 l of gas (n.u) was released. Determine the composition of the initial mixture in mass fractions. Methanol and ethanol inhibit the activity of the Central and peripheral nervous systems, methanol even in small quantities (5-10 ml) causes vision loss.
8.2. Deprived of three grams of iron from the body, a person would cease to exist. Hemoglobin-a compound of iron with protein-is a carrier of oxygen in the body. Magnesium in the human body is kept up to 19 g, magnesium ions form complexes in cells with nucleic acids. Magnesium is one of the main activators of enzymatic processes In the interaction of 10.4 g of a mixture of iron and magnesium with hydrochloric acid stood at 6.72 l of gas (n). Determine the mass of each metal in the mixture.
8.3. Silver nitrate has antimicrobial properties and is used in the treatment of skin ulcers, as well as lesions of the mucous membranes of the eyes (conjunctivitis) and larynx (laryngitis), it is used for cauterization of warts. To prove the presence of silver ions can be using halide ions. When silver nitrate was applied to a solution containing 6 g of a mixture of sodium and potassium bromides, 10.46 g of silver bromide was obtained. Determine the mass of each salt in the mixture.
8.4. Saponification of 5.6 g of a mixture of ethyl esters of acetic and formic acids required 25.96 ml. solution with a mass fraction of sodium hydroxide 10% (p = 1.08). Find the mass fraction of esters in the mixture. Esters with a small relative molecular weight often have a floral or fruity smell, they are used as flavorings in the food industry.
9. Energy.
The human body is a unique "chemical plant" in which a variety of chemical reactions occur, According to the law of conservation of energy, a person needs to maintain a certain amount of energy. Energy consumption, as you know, is replenished by power. The main components of food-carbohydrates, proteins, fats. As a result of digestion, these substances are converted into simpler ones and are carried by blood to all cells of the body, where they are oxidized by oxygen delivered by blood from the lungs. The end products of oxidation reactions are carbon dioxide and water, in parallel there is the formation of incomplete oxidation products, which are also excreted from the body. The process of oxidation of organic substances in the cells of the body is the main source of energy needed by the human. In accordance with Hess's law, the total thermal effect of the oxidation reaction does not depend on the reaction path (on its mechanism), that is, from the number and complexity of the intermediate stages, and is a constant for 9.1. Calculate how many degrees your body temperature would rise after a glass of sweet tea, if all the sugar that came with tea immediately oxidized in the body to carbon dioxide and water. In the calculations, it should be assumed that one teaspoon contains 10 g of sugar; the heat capacity of the body is equal to the heat capacity of water and is 4.2 kJ/(kg * K•; the thermal effect of the sucrose oxidation reaction is 5650 kJ/mole; human weight is 60 kg.
9.2. When burning 1 mole of glucose, 2816 kJ of heat is released. What is the energy reserve contained in 100g of 5% glucose solution?
9.3. At oxidation in a human body of fat weighing 1G 38,9 kJ of heat is allocated. Calculate how much heat is formed in the body of a person per month, if the daily rate of fat by weight is 106 g. each specific reaction.
9.4. To convert 1 molecule of carbon dioxide into glucose during photosynthesis, an average of 12 quanta of orange – red light are consumed. How many of these quanta will be spent on obtaining 1 kg of glucose?
9.5. Adenosine triphosphate, involved in the process of energy transfer in the organism, is a nucleotide constructed from adenine, ribose and a chain of three molecules of phosphoric acid (connected to each other by the scheme of formation of anhydride) and the structural formula of adenose-intraphosphate.
9.6. Why does the oxidation of fats produce more energy than the oxidation of starch?
9.7. Eating a bar of chocolate weighing 100g, a person receives 529 kcal (1 kcal = 4, 184 kJ). Calculate the mass of aluminum, which, reacting, produces the same thermal effect: 2Al + Fe2O3 = 2Fe + Al 2O3 + 854 kJ Chocolate is rich enough in oxidants that rejuvenates, prevents some diseases and preserves the beauty. It contains a lot of Nickel, an important derivative for hormones. Cocoa powder contained in chocolate reduces the risk of heart disease. All this applies to a greater extent bitter chocolate.
9.8. In a living organism, glucose is oxidized by oxygen of the air, and the released energy gradually accumulates in the cells in the form of ATP. Under the action of microorganisms, glucose molecules are able to split into smaller ones, which has found great application in the food industry and in agriculture. Determine the thermal effect of the chemical reaction a) oxidation b) alcoholic fermentation Glu-goat, if the heat of formation of glucose 1263,0 kJ / mole, alcohol 277,6 kJ / mole, water 285,8 kJ/mole, carbon dioxide 393, 5 kJ / mol.
9.9. Barite water-a saturated aqueous solution of barium hydroxide is used to absorb carbon dioxide. Give examples of possible practical applications of barite water. Calculate the thermochemical equation BaO + H2O = BA (OH) 2 + 73 kJ how much energy is released during hydration 30.6 g of barium oxide.
9.10. When organic substances rot, hydrogen sulfide (the smell of dead eggs) is formed. In a mixture with air it is explosive, poisonous. On the thermochemical equation of hydrogen sulfide oxidation 2h2s + 3O2 = 2 SO2 + 2 H2O + 1123 KZhD calculate the amount of heat that will be released:
a) at combustion of 3,4 g of hydrogen sulfide;
b) in the formation of 6.4 g SO2;
C) at combustion of 5,6 l (n. u.) hydrogen sulfide;
d) in the formation of 110 l SO2 (n. u.).
9.11. Tom chased Jerry for about 30 minutes until he realized he had no more strength left. How many grams of glucose consumed the muscles of his legs, if it is known that when running at an average speed, the muscles of the legs spend about 24 Kj (6 kcal) of energy in 1 minute?
10. Finding the molecular formula.
10.1. The average composition of the studied salts that contain cations of copper. It is believed that the deficiency of copper ions in the human body is the cause of cancer. The thermal decomposition of 1 mol of this salt produced 8 g of copper (II) oxide, 4.48 l of nitric oxide (IV), and 1.12 l of oxygen. Determine the molecular formula of the compound.
10.2 during thermal decomposition of 6.62 g of heavy metal (II) nitrate, the outflow of which causes poisoning of the human body, 1.12 l (n. u.) of a mixture of oxygen and nitric oxide was released. Determine the molar formula of metal nitrate.
10.3 burning 1.7 g of an unknown substance in oxygen produced 3.2 g of sulfur oxide (IV) and 0.9 g of water. Establish the formula of the substance, if it is known that it is lighter than argon. An aqueous solution of this substance is used to treat rheumatism and skin diseases. It is released by the decomposition of organic substances.
10.4. The combustion of 4.4 g of hydrocarbon produced 13.4 g of carbon monoxide (IV). This substance is used in everyday life, by determining its molecular formula, you can tell exactly where and how to handle it.
10.5. This substance is used in the production of drugs, but its vapor at high concentrations acts on the Central nervous system, liquid strongly irritates the skin. Determine its molecular (if possible, then structural) formula, if it is known that the combustion of 19.5 g of this hydrocarbon, formed 66 g of carbon dioxide. The relative density of its vapors in the air is 2.69.
10.6. When 5.4 g of unknown substance was burned, 1.8 g of water, 4.48 l of carbon dioxide and 2.24 l of nitrogen were formed. The vapor density of this substance in hydrogen is 13.5 . determine the formula of the substance. This is a strong poison, poisonous and salts that form this substance.
11. Qualitative tasks.
Qualitative tasks are tasks to determine the composition of the substance (which ions or functional groups are present in the composition of the substance), with the help of qualitative reactions, it is possible to determine the composition of mixtures. To solve qualitative problems, it is necessary to know the physical and chemical properties of the main classes of inorganic and organic substances, the reagents for determining the qualitative ion. Ions have different properties: they have a color, with certain other ions form precipitates of different colors and species, soluble in acids or alkalis, and this is based on their definition. When solving qualitative problems, you need to clearly represent the sequence of actions, this will help you design the work in the form of a table:
|
Используемые реактивы |
Исследуемые вещества |
Вывод |
||
|
NaOH |
Na2SO4 |
H2SO4 |
|
|
|
лакмус |
синий |
фиолетовый |
красный |
Пр. № 2 - H2SO4 Пр. №1- NaOH Пр. № 3 - Na2SO4 |
|
BaCl2 |
Нет изменений |
Белый осадок |
Белый осадок |
|
|
|
|
|
|
|
When recognizing substances it is necessary to remember:
- it is impossible to carry out experiments in a test tube with the investigated substance
- to consider whether it is possible to define to advantage of any one reagent
- if several substances are given, is it possible to do without the use of additional reagents.
- sometimes it is necessary to heat the substances, for which the test tube is fixed in the upper third of the holder, heated in an inclined position (the distance is directed away from the next sitting) in the upper part of the flame, without touching the bottom of the test tube wick.
11.1. In vitro solutions of medium salts with different color-ku: blue, light green (light green), yellow, which is determined by the cations of these salts it is Known that the solutions of these salts form a white precipitate with a solution of barium nitrate. Conduct appropriate experiments. Write down possible molecular formulas. Find out the value of cations for the human body.
11.2. In the study of solutions of unknown salts with the help of silver nitrate reactivation, the student received the following results:
Reagent-AgNO3
Test tube No. 1 White curd precipitate
Test tube No. 2 Light yellow curd precipitate
Test tube No. 3 Yellow curd precipitate
Test tube No. 4 no Changes observed
The composition of the salts includes a cation-antagonist to the action of sodium cation in the human body. Write down the formula of the studied salts. Prove the results of the study empirically.
11.3. Conduct a qualitative analysis of the barium chloride solution. As the property of barium ions to form a precipitate with sulfate ions is used in medicine.
11.4. Determine each of the three proposed substances without the use of additional reagents: zinc sulfate, sodium hydroxide, barium chloride. Make a plan for the study of substances. Which of these substances is used in medicine as a drug?
11.5. Determine the presence of Pb2+, Fe3+, Ca2+ions in the water sample. Using the table " the Role of ions in the human body, read about their meaning for humans. Lead ions are found in the muscle, bone tissue of the human body, as well as in the blood. But 1 mg is a toxic dose for the body, and a dose of 10 g is fatal.
11.5. Determine the presence of Pb2+, Fe3+, Ca2+ions in the water sample. Using the table " the Role of ions in the human body, read about their meaning for humans. Lead ions are found in the muscle, bone tissue of the human body, as well as in the blood. But 1 mg is a toxic dose for the body, and a dose of 10 g is fatal.
11.6. Baking bread often uses baking soda or ammonium bicarbonate. Prove their action empirically. Is it possible to use ammonium hydrosulfate for these purposes? The answer is confirmed by the equations of chemical reactions.
11.7. In three test tubes No. 1, No. 2, No. 3, there are salt solutions containing Ca2+, Na+ cations and CO32-, Cl-anions. Write down the possible formulas of the salts. Determine in which tube what substance. What role do these ions play in the human body?
11.8. In tubes there are solutions, each of which contains cations Ba2+, Zn2+, Na+, NH4+. Set in which of the tubes is the solution of each cation, using a solution of alkali. For a more correct definition, you can use one of the acids. Based on the fact that the three substances are used as drugs, assume which of the anions may be part of each solution.
11.9. Toxicity of heavy metals is explained by their ability to cause denaturation (destruction) of proteins. Explain why the toxic effect of heavy metal salts is higher the higher their solubility in water. How can the toxic effects of lead ions be confirmed experimentally?
11.10. In three test tubes are solutions of glycerol, glucose and formaldehyde. Recognize them with a single reagent. How these substances are used in human life.
11.11. Acetic acid is often used for preserving products. Is it possible to store canned food in galvanized or aluminum cookware? The answer confirm practical experience-mi.
11.12. Why olive oil is stored longer than sunflower?
11.13. How to identify old vegetable oil?
11.14. How, without breaking the egg, you can determine whether it is fresh or protein is found in it in the decomposition stage?
11. 15. How with the help of" lead " paper Pb (NO3)2 to determine whether the fish (meat) received for analysis is fresh or it has begun to deteriorate?
11.16. Why glycerin can be used as an additive in confectionery creams?
11.17. The human body always contains lactic acid, formed from glucose. With increased physical activity, its amount increases significantly, and part of it is released through the skin. A qualitative reaction to lactic acid can be a reaction with iron chloride (pale yellow staining). Make a wash from the skin of students before and after physical education lessons and confirm this statement. (1 ml flush + 2 drops of 1% p-RA iron chloride).
II. Non-standard tasks
1.What we eat. Most often, the chemical elements in the body come with food, you can calculate for yourself how much you need to eat certain foods to fill a certain rate of elements in the body.
Task
Solve the problem:
1. In 100 g of dried apricots contains 2,034 g of potassium. How many dried apricots do you need to eat to get a daily norm of potassium (1400-7400 mg.)?
2. In a piece of white wheat bread 0.8 mg of iron. How many pieces should be eaten per day to meet the daily requirement for this element) ?
3. A person needs 1G of calcium per day. How many glasses of milk should be drunk daily to supply the body with this element, if 1 glass of milk contains 280 mg of calcium?
4. The daily diet of an adult must necessarily include protein weighing 120g. the Mass fraction of protein in meat is 20%, in fish 18%, in cheese-34%. What is the mass of meat a person needs to eat in order to provide the body with a daily norm of proteins? Make a recount for t
5. The composition of pea fruits includes protein with a mass fraction of 26%, soy - 65%. These proteins contain all the acids the body needs. What weight of peas should be included in the diet to meet the daily requirement (120g) of the body in protein? Make a similar calculation for soybeans.
6. The daily human need for phosphorus is 1 g by weight. the Mass fraction of phosphorus content in food ( % ) in meat is 0.204, in eggs - 0.224, in cheese – 0.701. What is the mass of each product you need to enter into the diet to meet the daily needs of the body in phosphorus? h
7. The daily requirement of an organism for calcium makes 1 g. this requirement can be satisfied at the expense of milk. The mass fraction of calcium in cow's milk is 0.13%, and in goat's 0.14%. What weight of milk should be introduced into the daily diet to meet the needs of the human body in calcium? The results of research scientists have shown that daily intake of milk reduces the risk of tooth decaye fish and cheese.
8. Calcium orthophosphate is the mineral basis of bones and teeth. Other calcium compounds are involved in nervous and muscular activity, are part of the tissue fluid, nuclei and walls of cellular tissue of a living organism. Calcium reduces allergic re-actions. The daily requirement of an organism for calcium makes from 0,8 to 2 g. sources of calcium serve milk, kefir, cottage cheese, cheese, ry-BA, beans, parsley, green onions, and also eggs, buckwheat and oatmeal, carrots and peas. Will the daily requirement of the body in calcium, adding 1 g of calcium carbonate to food, provided it is fully absorbed?
9. Fluoride enters the human body with water and food. In some areas, the fluoride content in drinking water is 2 mg / l. calculate the mass of fluoride entering the human body daily, if he consumes about 2 liters of water per day. Write the dissociation equations of sodium fluoride and specify in what form fluoride enters the body. What can lead to excess fluoride in the body?
2. Vitamins
The term vitamin was proposed by the Russian scientist Lunin and is translated as amines of life (vitos-life). This emphasizes the importance of these compounds to ensure normal life. Amines are compounds containing nitrogen. Vitamins are essential nutrition factors of organic origin, present in food in small quantities, are not plastic material, but are involved in the regulation of biochemical and physiological processes. Vitamins and vitamin-like compounds include about 20 compounds, which are divided into two large groups. Fat-soluble vitamins (A, D, E, K) accumulate in the body, and water-soluble must be consumed constantly, as they are excreted from the body (C, b group, PP, folic and Pantothenic acids)
Not one biochemical process cannot do without vitamins, our organism cannot synthesize them independently, therefore vitamins constantly have to arrive in our organism. The cause of vitamin deficiency can be poor nutrition, physical or mental stress, work with harmful substances, metabolic disorders, acute chronic infectious diseases.
To avoid loss of vitamins it is necessary:
- consume as fresh food as possible;
- use less water for washing, soaking and cooking;
- it's better to eat vegetables raw;
- if possible, do not peel vegetables and fruits;
- do not pour, use vegetable broth-there are a lot of vitami-new;
- avoid long cooking of vegetables;
- prefer frozen products to canned, frozen will have more vitamins.
Solve the problem
1. Calculate how much of a lemon you need to eat every day in order to fill the body's need for vitamin C (50-100 mg). In the calculations, it should be assumed that the weight of a lemon is 100 g; the content of vitamin C (ascorbic acid) in a lemon is 0.5 %
2. Lack of nicotinic acid (vitamin PP) in the body causes lethargy, irritability, depressed mood. In clock his need for boys in age 15-18 years (in mg) numerically Rav-on 0.5 mol atoms calcium, and for girls roughly 0.2 mol atoms phosphorus. Calculate the daily requirement of vitamin PP. To replenish it in the body, eat buckwheat porridge, liver, kidneys, coarse bread, dates.
3. The content of vitamin C (in mg) in 100 g of cranberries is numerically equal to 0.1 mol of calcium carbonate. The daily requirement of vitamin C in the medium it 60 mg. How much cranberry is necessary to increase the daily requirements of the body's resistance to infections.
4. The daily requirement for vitamin D is 0.01 mg. Determine whether the norm of vitamin d intake will be met if 5 drops of 0.01% solution in oil are taken once a day. The volume of one drop is 0.04 ml, the density of the solution is 0.92 g / ml.
4. The transport system of the body-blood: Blood is a kind of tissue of the body, it serves as a "transport system" that carries the necessary nutrients and acid in the body. Consequently, it contains many different ions.
The metal content in the blood
|
Компоненты крови |
Содержание элементов в (мМ -миллимоль) |
|||||||||
|
s - элементы |
d – элементы |
|||||||||
|
Na+ |
K+ |
Mg2+ |
Ca2+ |
Mn |
Fe |
Co |
Cu2+ |
Zn2+ |
Mo |
|
|
Кровь (вся) Кровяные тельца Плазма (92% воды) Сыворотка |
85,2 20,9
141,3
140,1 |
44,5 94,9
4,11
5,06 |
1,57 2,72
1,13
0,87 |
- -
-
2,42 |
2,18 1,46
0,73
- |
8,59 18,6
-
23,3 |
0,71 _
_
- |
14,8 11,9
18,3
18,1 |
138,4 -
47,2
226,5 |
В кро-ви нет |
It turns out that the human body contains a certain amount of precious metals
|
Ме-талл |
Содержание в % |
Ежедневный прием с пищей |
Токсическая доза |
Летальная доза |
||
|
|
мышцы |
кости |
Кровь м/л |
|||
|
Ag |
(0,009-0,28) • 10-4 |
(0,01-0,44) • 10-4 |
< 0,003 |
0,0014-0,05 мг |
60 мг |
1,3-6,2 г |
|
Au |
- |
0,016 •10-4 |
0,1-4,2•10-4 |
- |
Неток-сичен |
|
Ascertained:
* when stress increases the content of zinc in the blood;
* shortly before a heart attack, the content of Nickel and manganese increases;
* atherosclerosis in venous blood increases the content of Mn and Fe and decreases the content of Ni and Co, and in arterial blood decreases the concentration of Zn, Mn, Cu, Co, Mo and increases the content of Pb, Cr, Al;
* in hypertensive crisis in the blood decreases Co and Zn; * diabetes mellitus is accompanied by a drop in Mn concentration in the blood;
* when pneumonia decreases the blood content of Co;
* it is believed that hard water has a negative impact on diseases of the cardiovascular system, apparently, the increased content of cadmium, calcium, magnesium and vanadium are unfavorable for heart activity;
• ischemic disease in the blood serum reduced the content of Zn;
* manganese, chromium, silver and zinc also have a positive effect on the heart, and the combined action of copper and titanium causes the opposite effect;
* Aggressive people have high levels of lead, iron, cadmium, calcium, copper and low levels of zinc, lithium and cobalt in their hair.
Task:
1. In the solutions section, look for problems related to the circulatory system and solve them.
2. Make up your tasks using the table on the content of metals in the blood.
3. In the study of chemistry, you operated with concepts such as substance, mixture, solution. Are they applicable in the treatment of blood?
4. Solved task. One drop of blood contains about 250 million red blood cells. Each red blood cell contains approximately 2.9 * 10-8 mg of hemoglobin. The molar mass of hemoglobin is about 67000 g/mol. Each hemoglobin molecule contains 4 iron atoms. What mass of iron can one drop of blood contain? How many hydrogen molecules does 1 hemoglobin molecule attach?
5. A little about a lot.
The mineral composition of the soil has a great influence on people's health:
* in the steppe regions of Ukraine in the soil reduced content of zinc, cobalt, iodine, which leads to myopia;
* low content of manganese, molybdenum, copper, chromium in the Krasnodar region in the soil is the cause of skin cancer;
* taiga-forest non-Chernozem zone of Russia is characterized by a lack of calcium, phosphorus, potassium, cobalt, copper, iodine, molybdenum, zinc, but an excess of strontium and manganese;
* in the forest-steppe and steppe Chernozem zone there is a lack of manganese, potassium, iodine, zinc and molybdenum are poorly balanced with other elements;
* dry-steppe, semi-desert and desert zones are characterized by high content of boron, zinc, sulphates, often strontium, but relatively low content of iodine, copper, sometimes cobalt.
Task:
4. After analyzing the composition of the soil discuss:
- how will the excess and lack of certain minerals affect the health of the people living there;
- what measures to take to prevent diseases;
- create tasks using this information.
6. Chemistry and hygiene
"A man is healthy as long as his teeth are healthy," the ancients said. Caries and periodontal disease are the most common dental diseases. According to statistics, only 3% of people are genetically not prone to these diseases, mainly people of the Negro race. They develop under the influence of microorganisms that produce lactic acid from food residues, especially coal-water. Any defect of tooth enamel (its composition is close to the mineral hydroxy-dapatite Ca5OH (PO4)3) leads to the penetration of microorganisms into the internal tissues of the tooth, which causes their inflammation (dentitis, pulpitis). The formation of lactic acid, which appears in the oral cavity during the distribution of food, especially carbohydrate, sharply reduces the pH of saliva from 7.0 -7.5 (neutral) to 4.5-5 (acidic). With such acidity, the destruction of the EMA-Li is accelerated, so it is always necessary to brush your teeth, tongue, and rinse your mouth after eating.
The main means of dental care – toothpaste. What does it consist of?
Toothpaste is a multi-component system that has the following composition:
1. Abrasives (most often used for these purposes, carbonate, calcium phosphate and hydrophosphate, silicon dioxide and titanium, aluminum oxide) carry out mechanical cleaning of teeth from plaque and polishing them;
2. Binders (usually substances extracted from seaweed or cellulose derivatives-sodium carboxy-methylcellulose) are used to turn a mixture of abrasive powders into a resistant paste;
3. Thickeners (glycerin, sorbitol, polyethylene glycol) are used to obtain a plastic, extruded from the tube mass, as well as they contribute to the preservation of moisture in the paste, improve taste;
4. Foaming agents (surfactants, e.g. sodium lauryl sulfate – CH3 (CH2) (OSO3Na) promote purification
5. Antiseptics (formaldehyde, chlorinated phenols, benzoic acid) eliminate germs;
6. Fluoride compounds translate Ca5OH (PO4)3 into CaF2 , and it, in turn, is adsorbed on the enamel, protecting it from the effects of acids, as well as fluoride compounds suppress the vital activity of bacteria, enamel is not destroyed.
7. Flavoring additives (saccharin and its derivatives) and fragrance (menthol, mint oils, clove oil, eucalyptol) give toothpaste a pleasant smell and taste.
Solve the problem
1. Poor dental care, especially untimely removal of food residues , is one of the causes of caries. Why is carbohydrate food (white bread, cookies, sugar) especially dangerous for teeth?
2. How much sodium monophosphate NaF * NaPO3 is contained in a tube of toothpaste weighing 100g, if the package contains information that the content of active fluoride is 0.15%?
3. Dentists recommend to use 1.5 g of active fluoride annually to prevent caries. How many tubes of toothpaste should be used per year to ensure this rate, if it is known that a tube weighing 100g contains 0.15 % of active fluoride?
4. On a tube of paste "Pepsodent plus" the composition is specified: sodium monophosphate, aluminum oxide, titanium dioxide, sodium lauryl sulfate, carboxymethylcellulose, flavoring substances, saccharin, benzoic acid. Explain what function each of the components performs?
Task:
1. Make:
a) crossword puzzle, using information about the composition of toothpaste
b) calculation tasks
2. Collect information about the composition of toothpastes sold in your stores, analyze it, choose the most convenient and convenient toothpaste.
3. Make a collection of toothpastes (tube boxes) for the chemistry Cabinet.
Скачано с www.znanio.ru
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.