
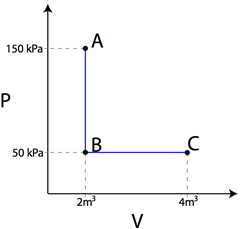
 Question: Using the PV diagram at right, find the amount of work required to
transition from state A to B, and then the amount of work required to
transition from state B to state C.
Question: Using the PV diagram at right, find the amount of work required to
transition from state A to B, and then the amount of work required to
transition from state B to state C.
Answer: The amount of work in moving from state A to B is equal to the area under the graph for that transition. Since there is no area under the straight line, no work was done. The work in moving from state B to state C can be found by taking the area under the line in the PV diagram.
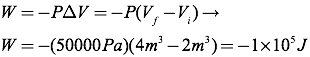 Note that the work is negative,
indicating the gas did work, which correlates with the gas expanding.
Note that the work is negative,
indicating the gas did work, which correlates with the gas expanding.
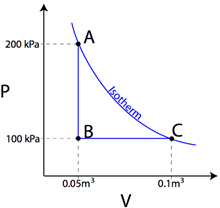 Question: Heat is removed from an ideal gas as its pressure drops from 200
kPa to 100 kPa. The gas then expands from a volume of 0.05 m3 to 0.1
m3 as shown in the PV diagram below. If curve AC represents an
isotherm, find the work done by the gas and the heat added to the gas.
Question: Heat is removed from an ideal gas as its pressure drops from 200
kPa to 100 kPa. The gas then expands from a volume of 0.05 m3 to 0.1
m3 as shown in the PV diagram below. If curve AC represents an
isotherm, find the work done by the gas and the heat added to the gas.
Answer: The work done by the gas in moving from A to B is zero, as the area under the graph is zero. In moving from B to C, however, the work done by the gas can be found by taking the area under the graph.
![]()
The negative sign indicates that 5000 joules of work was done by the gas. Since AC is on an isotherm, the temperature of the gas must remain constant, therefore the gas’s internal energy must remain constant. Knowing that ΔU=Q+W, if ΔU=0, then Q must be equal to -W, therefore 5000 joules must have been added to the gas.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.