
Worksheet
Thermodynamic Work
- describe ways to change the internal energy;
- determine the work on the schedule of pressure versus volume P(V)
- apply the formula of work in thermodynamics
(I) Task 1. Watch a video and answer the questions:
1. What is the process considered in the figure?
2. What letter represents work?
3. What is the work of gas?
4. What is the formula of work?
5. If the process is isochoric what is the work equal to?
(G) Task 2. Solve problems in pairs
1. 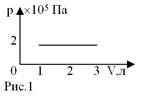 The volume of ideal gas increased from 1 L to 3 L (Fig. 1). Gas
work is equal to...
The volume of ideal gas increased from 1 L to 3 L (Fig. 1). Gas
work is equal to...
2. A gas bubble rises from the bottom of the reservoir. Does gas perform work? Assume constant temperature of the liquid in the reservoir.
3. Is it possible to transfer some heat to the body without causing a rise in its temperature?
4. Why do gases get hot during compression?
5. Gas, when expanding from a volume of 2 · 10-3 m3 to 6 · 10-3 m3, performed work 600 J. Determine at what pressure the expansion process was performed.
6.
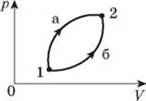 Gas can be transferred from state 1 to state 2 in two ways (see
fig.). In what case is a big work being done? During which process will the
change in the internal energy of the gas be greater?
Gas can be transferred from state 1 to state 2 in two ways (see
fig.). In what case is a big work being done? During which process will the
change in the internal energy of the gas be greater?
7. The gas that occupied the volume of 7 liters expanded to a volume of 33 liters at a constant pressure of 500 kPa. What work did the gas do?
8.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.