
1. A gas sample occupies a volume of 60 lt, under 100 kPa pressure. What is the volume of the same gas sample under 250 kPa pressure, at the same temperature?
2. A 24 lt container is filled with air at 100 kPa. Then it is connected with a 6 lt empty evacuated vessel. What is the final pressure of the combined system if the temperature stays constant?
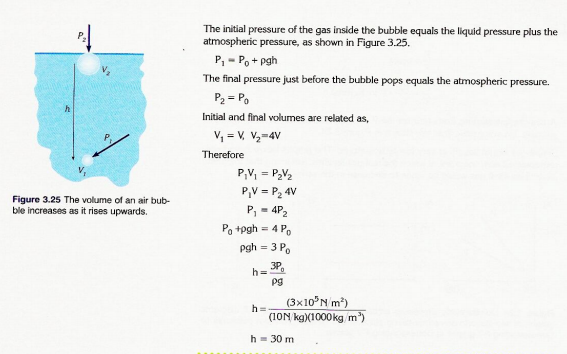
3. The volume of an air bubble becomes four times greater as it rises from the bottom to the top of a lake. What is the depth of the lake if its temperature is constant? Atmospheric pressure is measured to be 100 kPa and the density of water is 1000 kg/m3.
1. P1V1=P2V2 at T=const
60x100k = V2x250k
V2=24 lt
2. P1V1=P2V2
24x100k=(24+6)V2
V2=80 kPa
3,
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.