
Laboratory work 2: ‘Investigation of current conditions in electrolytes’
Objective: to define empirically the current conditions in electrolytes
Materials:
• Snap Circuits kit
• Batteries
• Styrofoam cup
• Salt
• Paperclip, steel bolt, or other small objects
Procedure
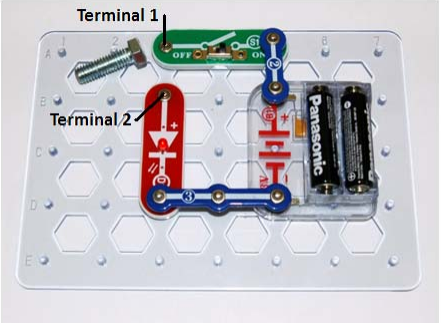
1. Set up the Snap Circuits so that you have the circuit shown below:
2. With the switch set to “on,” Place a bent paperclip across the terminals as shown in the
picture—the LED should light up.
3. Place any other objects you would like to test between the terminals marked 1 and 2 in the
diagram. Test materials like wood, metal, and plastic.
4. Connect the Snap Circuit wire leads to terminals 1 and 2. Fill the Styrofoam cup about
three‐fourths full with water, and submerge one end of each leads in the cup. Turn the switch to
 “on”
and note any changes in the LED.
“on”
and note any changes in the LED.
5. Turn the switch to “off,” and add 1 tablespoon of table salt to the cup, and mix until it’s
dissolved. Flip the switch and note any changes in the LED brightness.
Questions:
1. Which terminal is positive and which one is negative in the setup above? What could you do to quickly reverse the polarity of the terminals?
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
2. What is the voltage across the terminals? (Hint: look for the voltage of a single AA battery—the battery voltages add together.)
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
3. What types of materials caused the LED to light up? Did any of your materials make the LED light up only slightly?
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
4. Did the LED light up when you placed the wires in the regular water? What about after you
added the salt?
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
5. Salt water does not conduct electricity in the same way as metals, where free electrons can
move throughout the material easily. Instead, when sodium chloride dissolves in water its
molecules are broken up into charged particles called ions. Based on how much the LED lit up in
step 5, what can you conclude about how well the ions in the solution carry charge compared
with electrons in a metal conductor?
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.