
Mark Scheme
1 pressure decreased by 0.31 times the original pressure [1]
2 γ = 1.4 [1]
therefore it is diatomic [1]
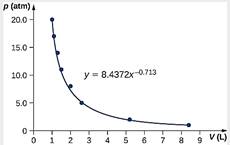 3
3
[1]
γ = 0.713 [1]
4 (a)
![]() [1]
[1]
T2 = 61 K [1]
(b) An adiabatic expansion has less work done and no heat flow, thereby a lower internal energy comparing to an isothermal expansion which has both heat flow and work done. [1]
Temperature decreases during adiabatic expansion. [1]
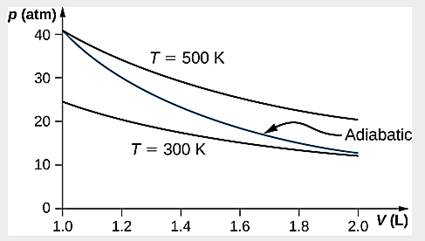 5
5
· More problems at:
https://cnx.org/contents/OFwJyVav@8/Adiabatic-Processes-for-an-Ideal-Gas
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.