
General chemical properties of metals-SUBSTANCES AND their PROPERTIES - LESSON PLANS for CHEMISTRY 11 class - lesson plans-lesson plans-author's lessons-plan-lesson summary - chemistry
The purpose of the lesson: to generalize, systematize and deepen students ' knowledge of the chemical properties of metals, while applying knowledge of the structure of metal atoms, their reducing ability according to a number of metal stresses, to teach them to correctly draw up equations of chemical reactions that confirm the properties of metals.
Basic concepts: electrochemical series of metal stresses, reducing capacity, intermetallic compounds, oxides , halides, sulfides, hydrides, nitrides, peroxides.
Equipment: Mendeleev PSCE, electrochemical series of metal stresses, a set of reagents (oxides, bases, acids, salts, indicators), nonmetals: S, O2; metals: Na, Mg, Zn, Fe, Cu, CH3COOH — acetic acid, C2H5IT is ethanol; a codoscope, test tubes, chemical glasses, alcohol lamps, a holder.
Lesson progress
I. Front-end survey
1. Where are the metals located in the PSE? What is the structure of their atoms? What families do these metals belong to (examples)?
2. What are the General physical properties of metals? What is the reason for the similarity of chemical properties?
3. On some physical properties of metals differ from each other? What is the reason? How are metals classified?
The answers to the questions are formulated by students based on notes in notebooks and the text of the textbook § 18.
At the end of the front-end survey, it is necessary to analyze the answers to questions 1, 2 § 18.
Alkaline and alkaline earth metals are located in groups I and II of the main subgroups. These are s-elements. Valence electrons are located at the external energy level, s-sublevel. In alkaline metals, ns1is one s-electron, so S-O +1; in alkaline earth metals, ns2is two s-electrons, S-O +2.
For metals of side subgroups, the d-sublevel of the pre-external energy level is filled in. Valence electrons are located at the s-sublevel of the external energy level andd-sublevel of the pre-external energy level. C. they manifest depending on which electrons participate in the formation of the compound. If s-electrons are involved, then the C. O. will be minimal. If you are s - and d - electrons, the S. O. can be maximum equal to the group number of the element, and intermediate.
II. Learning new material
Plan of presentation
1. Metals are reducing agents. Reducing capacity of metals based on their position in the PSE.
2. Electrochemical series of the stress of metals.
3. General chemical properties of metals:
a) interaction with simple substances — non-metals, metals;
b) interaction of metals with complex substances: water, acids, oxides and salts, organic compounds.
From the course of inorganic chemistry of the 9th class, some information about the chemical properties of metals is known. The main task of studying the secret question in the lesson is to generalize the basic information of the theory about the chemical activity of metals based on the structure of their volumes. In metal atoms at the external energy level, there are a small number of electrons and a very large atomic radius, which contributes to the rapid return of electrons by metal atoms as a result of their interaction with other compounds.
![]()
Metals are oxidized and act as reducing agents themselves. The chemical activity of metals can be estimated based on their Position in the PSE.
In periods — with an increase in the nuclear charge of the atom, it weakens; in groups, the main subgroups with an increase in the nuclear charge, it increases, because the larger the atomic radius and fewer electrons at the external energy level, the less energy is required for their separation.
However, most reactions occur in aqueous solutions. The chemical activity of metals will then be determined based on their position in the electrochemical series of metal stresses. In this series, metals are arranged taking into account the energy spent on the games of valence electrons and destruction of the crystal lattice, as well as taking into account the energy released by thehydrationof the ion ion on the metal.
Thus, less energy to spend on the gap and the gap of the crystal lattice and yields more energy when hydratesAI IOnew metal, the stronger reducing ability of metals in reactions in aqueous solutions, the left he is within range of stress of metals.
It should be noted that: high electrochemical activity of a metal does not always mean its chemical activity (and Vice versa). Pay attention to the location of Li and Na in the PSE and in the electrochemical series of metal stresses.
Based on their location in the PSE, Na is more active than Li (only one factor is taken into account — the atomic radius Ar(Na) > Ar(Li)). Based on the position in the electrochemical series of metal stresses , Li is located to the left of Na, its reducing capacity is higher, because in this case, not only the atomic radius of the metals is taken into account, but also the energy of electron separation, the energy of crystal destruction, and the energyии иоof metal hydrate ions. In General, there are three factors.
When working with the electrochemical series of metal stresses, one should remember:
- metals are arranged in descending order of reducing properties for reactions in solutions (t° = 25 °C, P = 1 ATM);
— the metal standing to the left displaces the metal standing to the right from the solutions of their salts;
— a metal standing in a series of stresses up to hydrogen displaces it from dilute acids (except HNO3 (sec. ), HNO3(conc.) and H2SO4 (conc.));
- metals that are in the range of stresses up to Alinteract with water to form alkalis and release hydrogen. The remaining metals interact under harsh conditions to form metal oxide and hydrogen;
— metals that are in the stress series behind hydrogen do not interact with water;
— based on the reduction capacity of metals in a series of stresses, metals can be divided into three groups by activity:
from Li to Al — very active metals;
from Al to H2-metals of average activity;
from H2 to Au-low-activity metals.
Knowing this conditional division of metals by their chemical activity, it is possible to correctly explain their chemical properties in relation to simple and complex substances.
1. Interaction of metals with simple substances-nonmetals and metals:
a) interaction with oxygen:
![]()
Experiment:
![]()
Peroxides and oxides are formed.
b) interaction with gray.
Experiment:
![]() —
zinc sulphide
—
zinc sulphide
Sulfides are formed.
C) interaction with the Halogens:
![]()
Mg + Cl2= MgCl2without heating.
Halides: fluorides, chlorides, bromides, and iodides.
d) interaction with hydrogen — only active metals:
2Na + H2= 2naoh-sodium hydride;
e) interaction with nitrogen:
6Li + N2= 2Li3N-without heating.
The other metals react with nitrogen when heated; these are nitrides.
f) interaction with metal, obtaining an intermetallic compound: Cu3Au, LaNi5.
2. Interaction with complex substances:
a) interaction with water.
Experiment:
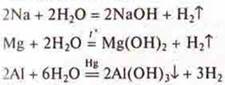 —
removal of oxide film under a layer of mercury,
—
removal of oxide film under a layer of mercury,
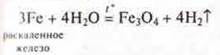
The more active the metal, the faster the reaction rate is.
b) interaction of metals with solutions of inorganic and organic acids.
Experiment: interaction of mg, Zn, Fe, СAnd u metals with solutions:
a) hydrochloric acid:
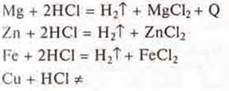
b) acetic acid:
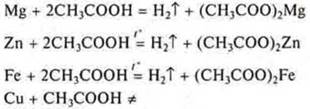
Based on the position of the metals Mg, Zn, Fe in the electrochemical series of metal stresses, we are experimentally convinced that they displace hydrogen from acid solutions. They are located before the hydrogen. Cu-does not displace hydrogen from acid solutions, since it is located in the electrochemical stress series behind hydrogen.
C) interaction of metals with solutions of salts.
Task: based on the position of metals in the electrochemical voltage series, justify the possibility of the following chemical reactions:
a) Zn and CuSO4; Withu and ZnSO4;
b) Fe and CuSO4; Withu and FeSO4.
Answer: a) Zn is more active Withu, because it is located in the stress series to the left of si; Zn is able to displace si from the solution of its salt.
Experiment (overhead projector):
![]()
b) Fe is more active Withu, since it is located in the stress series to the left of si; Fe is able to displace si from the solution of its salt.
Experiment:
![]()
When active metals interact (up to Al) with salt solutions, there is no displacement of the less active metal, because the active metal will displace hydrogen from the water.
C) interaction of metals with organic substances:
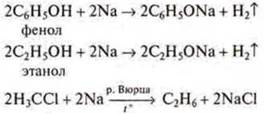
d) interaction of transition metals with alkali solutions.
![]()
III. Additional material
Many organic compounds contain metals. Such compounds are referred to as ORGANOMETALLIC. They are of great importance in organic synthesis.
Example: Grignard's reagent-CH3MgCl-is used in the synthesis of alkanes.
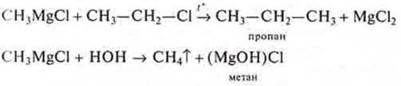
Connection Pb(C2N5)4-tetraethyl lead-used as an anti-detonator in motor fuel. This is a strong toxic compound. Gasoline is often called "leaded".
IV. Generalizations and conclusions
Knowledge of the specific features of the position of metals in the Mendeleev PSC, the ability to characterize the General chemical properties of metals: their relation to simple and complex substances in the electrochemical series of fins stresses.
V. Homework assignment
§ 18. up to p. 201-207; p. 223; 1) № 5 (y), № 6 (y); 2) № 8. № 9.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.