
Oxides and hydroxides of metals-SUBSTANCES AND their PROPERTIES - LESSON DEVELOPMENTS in CHEMISTRY 11 class - lesson developments-lesson developments-author's lessons-plan-lesson summary - chemistry
Lesson objectives: learn how to draw up the formulae of the oxides and hydroxides of the metals, compare their properties, given the situation in PSHE D. I. Mendeleev, the degree of oxidation and the radii of the ions; to consolidate knowledge of properties of oxides and hydroxides formed by metals of auxiliary groups; to give an idea of the long-period periodic table and the appearance of shortened.
Basic concepts: oxides, hydroxides, oxide amphotericity, hydroxide amphotericity.
Equipment: AlCl3, NH3, H2O, CON.
Lesson progress
I. Discussion of homework questions
№ 5 § 18.
When determining the position of a metal in a series of stresses, not only the energy of electron separation from individual atoms is taken into account, but also the energy spent on the destruction of the crystal lattice, as well as the energy released duringhydration of ions.
Example: lithium is more active in aqueous solutions than sodium (in PSE, sodium is more active by position than metal). The fact is that the energy ofии иоhydration of Li+ ions is significantly greater than the energy of hydration of Na+ ions, so the first process is energetically more profitable.
№ 6 § 18
The most active is K, because It is located to the left of all the proposed metals in the series of metal stresses. It is a stronger reducing agent.
№ 8 § 18
Chromel is completely soluble in dilute sulfuric acid (Ni, Cr, Co, Fe). All metals in the metal stress series are located to the left of hydrogen and displace it from dilute acids.
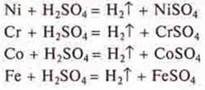
II. Test
|
Option I |
Option II |
|
1. Metal element. a) S; b) VA; C) Al. |
1. The transition element: a) Zn; b) P; C) Na. |
|
2. A metal that can displace hydrogen: |
|
|
out of the water: a) Withu; b) Li; C) Pb. |
from an acid solution: a) CA; b) Ag; C) Hg. |
|
3. From a solution of copper sulfate salt (II) |
|
|
copper can be displaced. |
copper is not displaced: |
|
a) Zn; b) CA; C) Fe; d) Na; e) Ag. |
|
|
4. Will the sample of the metal mixture |
|
|
Al, Mn, si dissolve in the solution? a) HCl; b) CON. |
Fe, Ag, Zn in solution: a) H2SO4; b) CON. |
|
Write the equations of reactions. |
|
Answers to the test questions
|
Option I |
Option II |
|
1) b; 2) b; 3) "a", "b"; 4) a) in NSl;
|
1) "a"; 2) "a"; 3) "b", "g", "d"; 4) a)
|
III. Independent work
|
Option I |
Option II |
|
1. Make up no more than five reaction equations that characterize the properties |
|
|
of barium. |
copper. |
|
2. To explain the possibility of chemical reactions (hydrolysis of salt is not taken into account): |
|
|
|
|
Answers to self-study questions
|
Option I |
Option II |
|
1. Ba is a very active metal:
|
1. Cu-low-activity metal:
|
|
2.
Al stands in a series of stresses to the left of hydrogen, able to displace it from the acid solution. |
2.
The reaction is possible, since lead is located in the stress series to the left of copper, its reducing capacity is greater than copper. |
IV. Learning new material
Plan of presentation
1. Oxides formed by metals, features of their properties depending on the S. O. of the element and the radius of the ion; changes in the properties of oxides of elements in periods and in groups, major subgroups.
2. Hydroxides formed by metal oxides; change in the character of the hydroxide depending on the S. O. of the element and the radius of its ion.
3. Character of oxides and hydroxides formed by metals of secondary subgroups. PSE is long-period and shortened.
All metals form salt-forming oxides. However, some oxides exhibit the properties of basic oxides, while others exhibit acid-base, i.e. amphoteric, properties. What does it depend on? It is necessary to know the following-the metal exhibiting the S. O. +1, +2 forms oxides of the basic character, since these are typical metals that are located in groups I and II, the main subgroups.
Example: 1 group, the main subgroup is represented by elements Li, Na, K , Rb, Cs, Fe. By the end of the main subgroup group, the atomic radius of elements increases. When the electron returns from the external level, cations are formed. S. o. all elements do not change by the end of the group, but the character of oxides — the main one, will increase,
![]()
The main character is strengthened
![]()
The radius of the new element increases
If we consider the change of properties of oxides of elements in period on the example of elements of period III, it should be noted that atoms of these elements the number of energy levels the same, but the modified S. O. By the end of the period, it increasesthat causes the decrease of ion radius. As a result, the oxide character changes from basic through amphoteric to acidic.
![]()
S. O. increases
![]()
The radius of the ion decreases, the nature of the oxide changes from basic to amphoteric
The
metal oxides correspond to the hydroxides. If the S. O. of metals +1, +2, +3,
+4, then they form oxides: ![]() and
hydroxides:
and
hydroxides: ![]()
The character of the hydroxide also depends on the S.O. of the element and the ion radius. The larger the PH, the smaller the radius of the ion, the character of the hydroxide is more acid-base, that is, it shows amphotericity:
in the period:
Example: period III.
Hydroxides: ![]()
Weakening of the base property, strengthening of the acidic property
![]()
Increases PH: decreases the radius of the ion; alkali, base, amphoteric base.
in groups, major subgroups:
Example: group I, the main subgroup.
![]()
S. O. does not change
![]()
The ion radius increases, and the properties of hydroxides as bases are enhanced
Transition elements located in short periods areVe (II period), Al (III period), form oxides BeO, Al2O3 and hydroxidesIne(HE)2 and Al(ON)3-showing amphoteric acid-base properties. What does this mean?
Experiment
Al(OH)3: a) preparation, b) acid-base properties.
Main properties: interaction with acids, oxides and hydroxides.
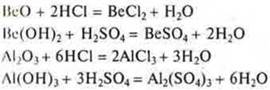 (experiment)
(experiment)
Acidic properties: interaction with alkalis and oxides, and hydroxides.
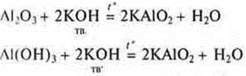
and if the solution is alkaline, then a complex is formed
![]() (experiment)
(experiment)
So, with an increase in the S. O. of metal, the radius of the metal ion decreases, the properties of metal oxides and hydroxides change from basic to acid-base, and then show an acidic character.
In the elements of side subgroups, the change in S. O. can be considered on the example of a single metal.
Example: chrome metal can exhibit a +2, +3, +6 CP.
![]() —
basic oxide, hydroxide Withr(OH)2— the base;
—
basic oxide, hydroxide Withr(OH)2— the base;
![]() -
amphoteric oxide, hydroxide Withr(OH)3-amphoteric base;
-
amphoteric oxide, hydroxide Withr(OH)3-amphoteric base;
![]() —
acid oxide, hydroxide H2CGO4, H2CG2O7-acid.
—
acid oxide, hydroxide H2CGO4, H2CG2O7-acid.
We
know that the maximum CPI is determined by the group number. The chrome element
is located in group VI, a side subgroup. The sulfur element is also located in
group VI, only the main subgroup. In the highest degree of oxidation they form
acid oxides![]() which
correspond to the acids H2CGO4and H2SO4.
Knowledge of such similarity of many elements allowed D. I. Mendeleev to build
a shortened table of chemical elements, where, in contrast длиннопериоднойto
the long-period group, the main and secondary subgroups appeared in addition to
the groups. All elements of the same group exhibit maximum valence, Which is
correspondingly equal to the group number.
which
correspond to the acids H2CGO4and H2SO4.
Knowledge of such similarity of many elements allowed D. I. Mendeleev to build
a shortened table of chemical elements, where, in contrast длиннопериоднойto
the long-period group, the main and secondary subgroups appeared in addition to
the groups. All elements of the same group exhibit maximum valence, Which is
correspondingly equal to the group number.
Let's go back to the example with chrome.
![]() —
oxides
—
oxides
![]() —
hydroxides
—
hydroxides
1) What happens to S. O.?
Answer: S. O. is increasing.
2)How do you think the ion radius changes?
Answer: the radius of the chromium ion decreases with increasing PH.
3) How do these changes affect the properties of the compound?
Answer: there is a change in the properties of oxides and hydroxides from basic, through amphoteric to acidic.
V. Generalizations and conclusions
1. All typical metals correspond to oxides of the basic character, and their hydroxides-bases;
2. Transition metals d-elements form several oxides and hydroxides. The properties of these compounds change depending on the change of the SN and the radius of the ion. With an increase in C. O. the radius of the ion decreases, the main properties weaken, acid-base increases, and then acidic both in oxides and hydroxides.
3. In one group of PSHE D. I. Mendeleev, but in different subgroups, there are elements where the valence maximum. S. O. respectively equal to the group number.
VI. Homework assignment
§ 18, p. 206-207. № 4, 10 p. 223.
VII. Pinning
Task: Make a formula for chromium (III) oxide and hydroxide. Equations of reactions confirm their amphotericity.
Answer: SG2O3is chromium (II) oxide.
![]() —
aboutthe sleep oxide interaction with acid.
—
aboutthe sleep oxide interaction with acid.
![]() -
кketotic oxide of interaction with alkali.
-
кketotic oxide of interaction with alkali.
![]() -
chromium (III) hydroxyl.
-
chromium (III) hydroxyl.
![]() — the
sediment disappears.
— the
sediment disappears.
Chromium hydroxide is a base that reacts with acid.
![]() — the
sediment disappears.
— the
sediment disappears.
Chromium hydroxide exhibits acidic properties, since it interacts with solutions of alkali.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.